Unveiling Magnesium: Its Atomic Number And Significance
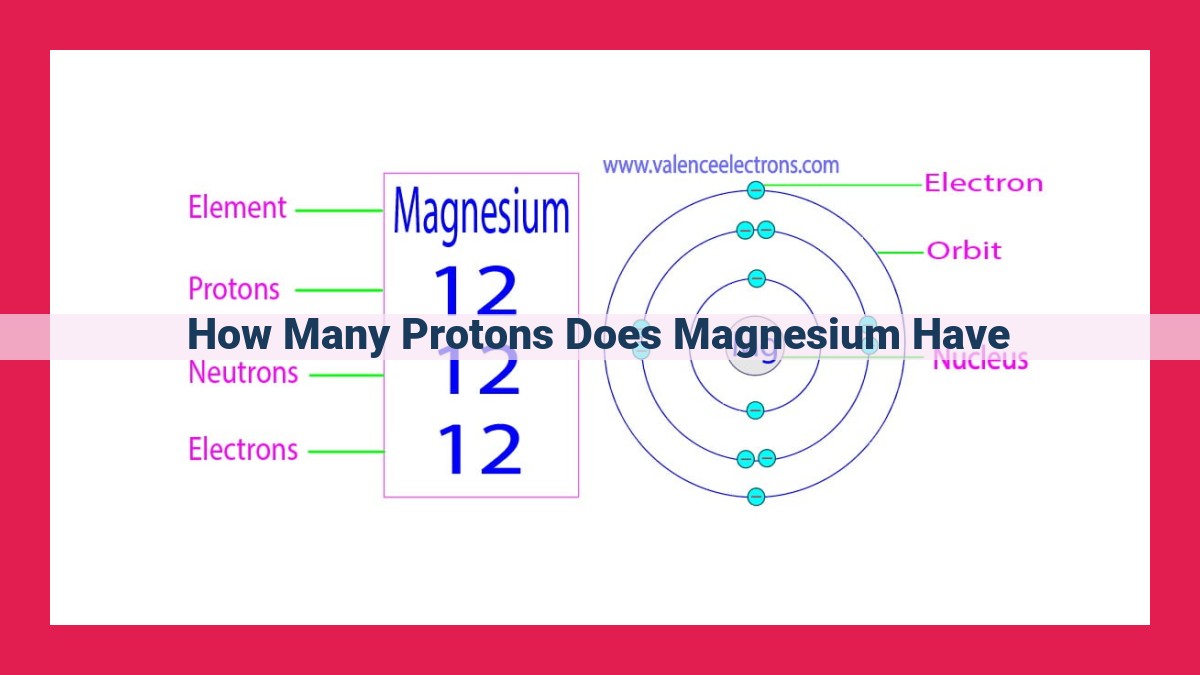
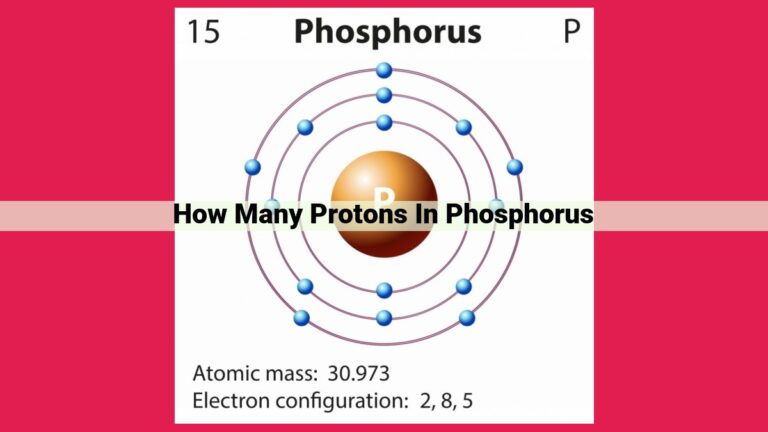
Magnesium’s atomic number, denoted by 12, reveals the presence of 12 protons within its nucleus. As protons are the defining characteristic of elements, this count establishes magnesium as an element with 12 protons, designated by the elemental symbol Mg. The atomic number of an element determines its position on the periodic table, where magnesium resides as the 12th element.
Understanding Atomic Number: The Keystone of Proton Count
In the captivating realm of chemistry, the concept of atomic number holds the key to unraveling the secrets of matter. Atomic number, a fundamental characteristic of every atom, unveils the very essence of an element, defining its identity and place within the periodic table.
At the heart of an atom lies its nucleus, a compact realm teeming with subatomic particles. Amidst this microscopic universe reside protons, positively charged particles that form the backbone of an atom’s nucleus. The atomic number of an atom, expressed as a whole number, corresponds precisely to the number of protons within its nucleus.
The atomic structure of an atom is a delicate balance of protons, neutrons, and electrons. Neutrons, neutral in charge, provide stability to the nucleus, while electrons, negatively charged particles, populate the atom’s outer shells. The harmonious interplay between these particles determines the atom’s unique properties.
By unraveling the atomic number of an element, we gain a profound understanding of its chemical behavior. As the number of protons increases from element to element, so does the number of electrons required to achieve electrical neutrality. This subtle dance between protons and electrons governs the element’s position on the periodic table, revealing its chemical characteristics and reactivity.
Example: Magnesium’s Atomic Profile
Let’s delve into the atomic profile of magnesium, a fascinating element with an atomic number of 12. This intriguing number signifies the presence of 12 protons within the magnesium nucleus. Its elemental symbol, Mg, succinctly represents this atomic essence.
Magnesium, with its 12 protons and 12 electrons, occupies a prominent position in the second row of the periodic table. Its unique atomic number grants it the ability to form stable bonds with other elements, contributing to its diverse applications in various industrial and biological processes.
Protons: The Cornerstones of Elements
In the vast tapestry of the atomic realm, protons reign supreme as the fundamental building blocks that define the very essence of elements. These subatomic particles, each carrying an unyielding positive charge, reside within the heart of every atom, forming the nucleus alongside their enigmatic counterparts, the neutrons.
The atomic number, a pivotal concept in chemistry, unveils the captivating tale of protons within an atom. It represents the invariable count of protons, like a fingerprint that distinguishes one element from another. This fundamental characteristic shapes the arrangement of elements on the iconic periodic table, orchestrated by the brilliant minds of Dmitri Mendeleev and Henry Moseley.
Each element, with its unique atomic number, assumes its rightful place on this grand stage. Carbon, with its atomic number of 6, hosts six protons in its nucleus, while oxygen, with eight protons, dances upon the periodic table with an atomic number of 8. This celestial ballet paints a vivid tableau of the elements, each with its own distinctive protonic symphony.
The atomic number plays a pivotal role in defining the elemental identity of a substance. It determines the number of electrons that orbit the nucleus, maintaining a delicate balance of positive and negative charges that endow each element with its characteristic properties. This intricate dance of subatomic particles dictates the chemical reactivity, physical states, and myriad applications of the elements that shape our world.
In the grand tapestry of the universe, protons emerge as the silent architects, subtly weaving the fabric of our existence. They lay the foundation for the periodic table, guiding us through the labyrinth of elements that surround us. By unraveling the secrets of protons, we unlock a deeper understanding of the fundamental principles that govern our world.
Unraveling the Proton Count: Magnesium’s Atomic Number
In the vast world of chemistry, elements are the building blocks of matter, each characterized by a unique set of properties. One crucial factor that defines an element’s identity is its atomic number, a number that governs its proton count. Let’s delve into this concept and unravel the atomic number of magnesium, an essential element renowned for its versatility.
Atomic Number: The Basis of Proton Count
Every atom comprises a tiny nucleus, the heart of the atom, where protons and neutrons reside. These subatomic particles play a pivotal role in determining an element’s character. The atomic number, denoted by the symbol Z, represents the number of protons an atom possesses. This number is unique for each element and determines its place on the periodic table.
Protons: The Foundation of Elements
Protons are positively charged particles with a mass approximately 1836 times greater than that of electrons. They reside within the nucleus, where their presence balances the negative charge of electrons orbiting the nucleus. The atomic number of an element is equivalent to the number of protons in its nucleus.
Magnesium’s Atomic Number: 12 Protons
Magnesium, a silvery-white metal, has an atomic number of 12. This means that every magnesium atom contains 12 protons in its nucleus. The elemental symbol for magnesium is Mg, and its atomic number is what distinguishes it from all other elements.
The atomic number is a fundamental property that defines the essence of an element. Magnesium, with an atomic number of 12, possesses 12 protons in its nucleus, establishing its unique identity. This number is crucial in determining magnesium’s chemical behavior, reactivity, and the properties that make it an indispensable element in various industries and applications.