Lithium: The First Basic Metal On The Periodic Table
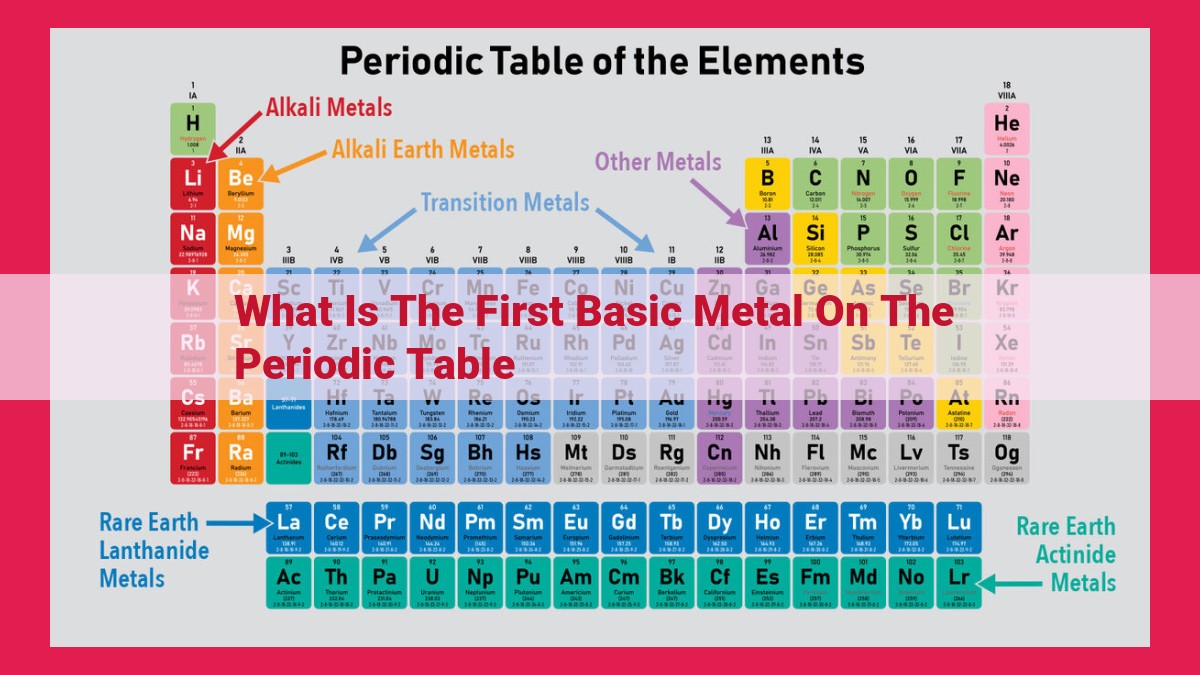
The first basic metal on the periodic table is lithium (Li). Basic metals are those that readily lose electrons to form positive ions, and they are characterized by their high reactivity and low melting points. Lithium is the first element in Group 1 (alkali metals), which are the most reactive metals. It has an atomic number of 3, with a single electron in its outermost shell. This electron is easily lost, giving lithium a constant oxidation state of +1 in compounds. Lithium’s high reactivity makes it an important component in batteries and other electronic devices.
Basic Metals
- Define basic metals and their characteristics
- Explain the relationship between basic metals and metals
- Highlight the significance of alkali metals as basic metals
Basic Metals: A Journey from Alkali Metals to Lithium
In the vast universe of elements, metals play a pivotal role, shaping our lives with their remarkable properties. Among these metallic wonders, basic metals stand apart, showcasing distinctive characteristics that have captivated scientists and engineers alike.
Basic metals are a family of elements that share a unique bond: they readily react with acids to form salts and liberate hydrogen gas. This reactivity, stemming from their loosely bound outermost electrons, sets them apart from other metals. Their tendency to lose electrons makes them electropositive, a property that underpins their exceptional chemical behavior.
Within the realm of basic metals, alkali metals hold a prominent position. These highly reactive elements are the sentinels of the periodic table, occupying the first column. From lithium, the lightest of all, to francium, the heaviest, they exhibit a striking pattern of increasing reactivity as we descend the column. This trend arises from their progressively larger atomic radii and more loosely bound outermost electrons.
Lithium: The First Alkali Metal
At the cusp of the periodic table, hydrogen, the loneliest of elements, stands alone in its first position. However, closely following in its wake is lithium, the inaugural member of the alkali metal family. Lithium, with its atomic number of 3 and chemical symbol Li, embodies the quintessential characteristics of alkali metals.
Like its heavier alkali metal brethren, lithium boasts a single, outermost electron that it readily relinquishes, granting it a constant oxidation state of +1. This electron configuration, expressed as 1s²2s¹, dictates its remarkable reactivity and its pivotal role as a reducing agent in various chemical processes.
Lithium’s atomic number of 3 signifies its place in the periodic table, determining its unique set of properties and distinguishing it from all other elements. This atomic number plays a crucial role in organizing the periodic table, with elements arranged in columns based on their shared number of electrons in their outermost energy level.
As the lightest alkali metal, lithium exhibits intriguing contrasts. It is the least dense of all solids, with a silvery-white appearance. Yet, it possesses a surprising strength and a high thermal conductivity. These properties, coupled with its low reactivity compared to other alkali metals, make lithium a valuable asset in various industries, including energy storage, aerospace, and electronics.
Unveiling the First Element: Hydrogen, the Pioneer of the Periodic Table
In the realm of chemistry, the periodic table reigns supreme as an indispensable guide to the elements that make up our world. Arranged in a meticulous order, each element holds its unique place, revealing its properties and characteristics. At the very dawn of this majestic table lies hydrogen, the unsung hero that occupies the prestigious position of the first element.
Hydrogen: The Foundation of the Periodic Table
Hydrogen, with its atomic number of 1 and symbol H, stands as the primordial element upon which the periodic table is founded. Its simplicity belies its profound importance, as it serves as the building block for countless molecules and compounds that shape our very existence.
Hydrogen’s Atomic Makeup
Delving into the atomic structure of hydrogen, we find a single proton at its core, orbited by a lone electron. This elementary configuration provides the foundation for understanding the chemical behavior of hydrogen, explaining its ability to bond with other atoms and form diverse compounds.
A Versatile Element with Far-Reaching Significance
Hydrogen’s influence extends far beyond its position on the periodic table. Its versatility shines in its abundance throughout the universe, playing a crucial role in the formation of stars, planets, and even the water that sustains life on Earth. From fuels to fertilizers, pharmaceuticals to plastics, hydrogen’s applications span a vast array of industries and enrich our daily lives in innumerable ways.
Embracing Hydrogen’s Legacy
As we appreciate the foundational role that hydrogen plays in chemistry and beyond, let us celebrate its pioneering spirit. This unassuming element serves as a constant reminder of the intricate tapestry of the periodic table and the enduring power of scientific discovery.
The First Alkali Metal: Lithium
In the realm of chemistry, the periodic table reigns supreme, organizing the known elements in an intricate dance of properties and characteristics. Amidst this tapestry of elements, the alkali metals stand tall as a family of highly reactive elements. They are so reactive, in fact, that they are never found uncombined in nature.
Of the alkali metals, lithium holds a special place as the first in line on the periodic table. It’s the lightest of all metals, making it a key player in battery technology for its ability to store energy in a compact form. But lithium’s story doesn’t end there.
Origins and Relationship with Hydrogen
The periodic table arranges elements based on their atomic number, which is a measure of the number of protons in an atom’s nucleus. Lithium has an atomic number of 3, indicating three protons and three electrons. This places it right after hydrogen, the first element in the table with an atomic number of 1.
Despite its position on the table, lithium bears a curious relationship with hydrogen. Hydrogen, the simplest element in the universe, contains only one proton and electron. As the second element, lithium adds two more protons and electrons to the mix, resulting in a chemical configuration that resembles hydrogen with an extra electron.
This shared electron configuration hints at a deeper connection between lithium and hydrogen. They both belong to a class of elements known as s-block elements, which have their outermost electrons in the s orbital. This outermost electron configuration gives them similar chemical behavior, such as their reactivity and tendency to form positively charged ions.
As the first alkali metal on the periodic table, lithium occupies a pivotal position in the study of chemistry. Its unique electron configuration, low atomic number, and relationship with hydrogen make it a fascinating subject for both scientists and students alike. From its use in batteries to its role in understanding the nature of elements, lithium continues to captivate and inspire in the world of science.
The Atomic Number of Lithium: Unraveling the Periodic Table’s Secrets
In the realm of chemistry, the periodic table stands as a roadmap to the elements, guiding us through their properties and behaviors. Each element occupies its own unique position, assigned based on its atomic number, a fundamental characteristic that holds the key to unlocking its identity.
Atomic number refers to the number of protons in the nucleus of an atom. Protons carry a positive charge, and their presence defines the element’s identity. For instance, all atoms with three protons are lithium atoms, regardless of their neutron count.
The periodic table is organized according to atomic number, with elements arranged in increasing order from left to right. This arrangement reveals patterns in the elements’ properties. Elements within the same column, or group, share similar chemical characteristics, while those in the same row, or period, typically have the same number of electron shells.
Lithium, the first element in Group 1 (or IA), has an atomic number of 3. This means that every lithium atom has three protons in its nucleus. The atomic number of lithium has profound implications. It determines the element’s chemical behavior, influencing its reactivity, bonding preferences, and ability to form compounds.
Understanding the atomic number of lithium is crucial for comprehending its position within the periodic table and its role in various chemical reactions. By unraveling the secrets of atomic number, we unlock a deeper understanding of the fundamental building blocks of our universe.
Lithium: The First Alkali Metal and Its Unique Symbol
The periodic table, a cornerstone of chemistry, provides a systematic organization for the elements. Starting with the lightest and progressing to the heaviest, these elements exhibit distinct properties and behaviors that define their place in the table. Among the first in this sequence is lithium, the element with the atomic number 3 and the symbol “Li.”
Lithium’s symbol, “Li,” holds a profound significance that ties it back to the origins of chemistry. The name “lithium” itself is derived from the Greek word “lithos,” meaning “stone.” This etymology stems from the fact that lithium was initially discovered in mineral ores, particularly in the mineral petalite.
The symbol “Li” was first proposed by the renowned Swedish chemist Jöns Jakob Berzelius in 1813. He derived the abbreviation from the Latinized form of the Greek name, “lithium,” utilizing the first two letters, “Li,” to create a concise and memorable symbol. This convention of using the first one or two letters of the element’s Latin name to form its symbol became a standard practice in chemistry.
The symbol “Li” not only serves as a convenient way to represent lithium but also embodies the element’s unique characteristics. As the first alkali metal in the periodic table, lithium possesses highly reactive properties, readily forming positive ions with a charge of +1. This reactivity is attributed to lithium’s electron configuration, which consists of two electrons in the first energy level and one electron in the second energy level. The lone electron in the outermost energy level is easily lost, leading to the formation of positively charged lithium ions.
Furthermore, lithium’s symbol holds importance in its use in various chemical formulas. For instance, the formula “LiCl” represents lithium chloride, a compound formed from the reaction of lithium with chlorine. The “Li” symbol denotes the presence of a lithium atom, while the “Cl” symbol represents a chlorine atom. By understanding the symbolism of chemical elements, scientists can effectively communicate the composition and structure of compounds, facilitating the exploration of their properties and applications.
Electron Configuration of Lithium
- Define electron configuration and its importance
- Determine lithium’s electron configuration (1s²2s¹)
- Explain how electron configuration influences chemical properties
Defining Electron Configuration
Picture your atom as a miniature solar system, with a nucleus at the center bustling with protons and neutrons. Orbiting this nucleus are electrons, represented by tiny particles. The electron configuration describes how these electrons are arranged in different energy levels or shells. Understanding electron configuration is crucial because it influences an element’s chemical behavior.
Lithium’s Electron Configuration
Our focus today is on lithium, an alkali metal found in the first column of the periodic table. Like all atoms, lithium’s electrons occupy specific shells. Its electron configuration, denoted as 1s²2s¹, tells us that:
- The first shell (1s) is filled with two electrons.
- The second shell (2s) has a solitary electron.
Electron Configuration and Chemical Properties
The electron configuration of an element plays a pivotal role in determining its chemical properties. In the case of lithium, its single electron in the outermost shell makes it highly reactive. This electron can easily be lost, making lithium a good reducing agent and a highly reactive metal. Additionally, lithium’s electron configuration explains its tendency to form ionic bonds with electronegative elements.
By understanding the electron configuration of lithium, we gain valuable insights into its chemical behavior. This knowledge serves as the foundation for exploring the properties and reactions of this fascinating alkali metal.
Oxidation State of Lithium in Compounds
- Explain the concept of oxidation state
- Determine lithium’s constant oxidation state of +1
- Discuss the role of oxidation state in forming chemical bonds
Oxidation State of Lithium in Compounds
When atoms combine to form compounds, they can undergo oxidation or reduction. Oxidation refers to a loss of electrons, while reduction indicates a gain of electrons. The oxidation state of an atom represents the hypothetical charge it would have if all its bonds were ionic.
In the case of lithium, it always exhibits an oxidation state of +1 in compounds. This means that lithium atoms tend to lose one electron when they form chemical bonds. The constant oxidation state of lithium is due to its unique electron configuration.
Lithium has three electrons, with two in the first energy level and one in the second energy level. The lone electron in the second energy level is loosely held, making it easy for lithium to lose this electron and achieve a stable octet configuration.
The oxidation state of lithium plays a crucial role in determining the type of chemical bonds it can form. By losing one electron, lithium ions have a positive charge that attracts negatively charged ions, such as chloride ions (Cl⁻), to form ionic bonds. The formation of ionic bonds between lithium and other elements is essential for the stability and properties of lithium compounds.
Understanding the oxidation state of lithium is fundamental in predicting its chemical behavior and explaining the properties of lithium-containing compounds. It allows scientists to comprehend the bonding interactions and reactivity of lithium in various chemical environments.