How To Calculate Liquid Mass Accurately: A Comprehensive Guide
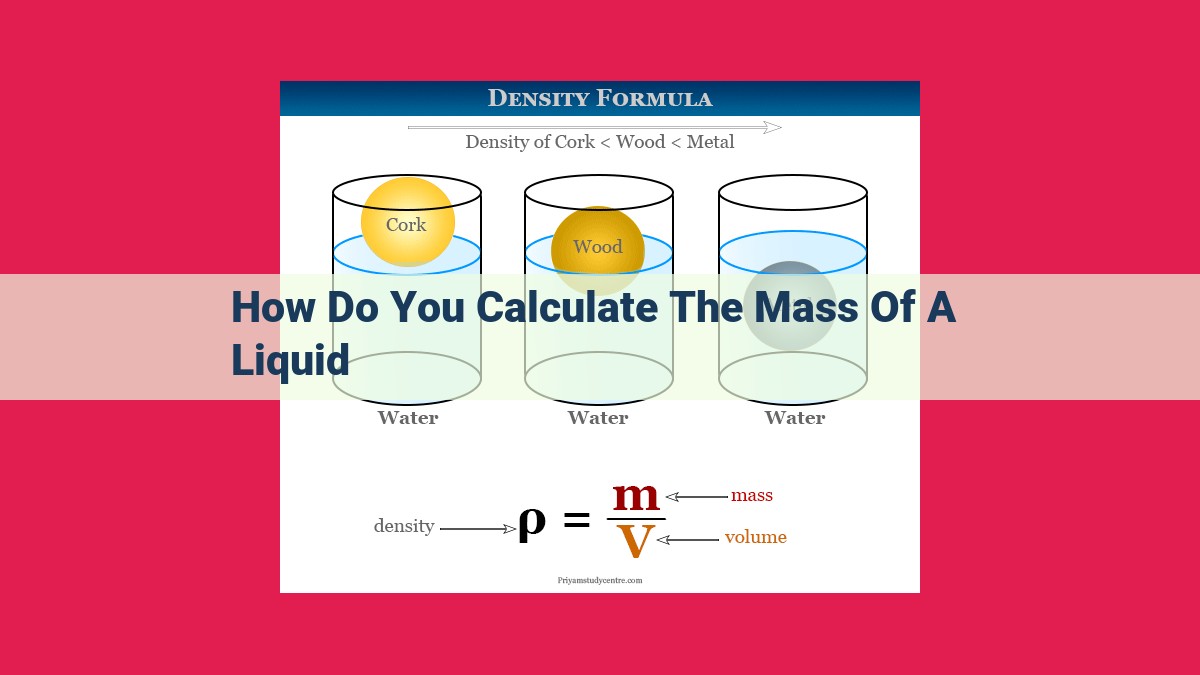
To calculate a liquid’s mass, determine its density, which represents the mass per unit volume. Measure the liquid’s volume accurately using graduated cylinders or beakers. Find the density value for the liquid from tables or databases, considering the liquid’s temperature and composition. Use the formula Mass = Density × Volume and substitute the measured volume and obtained density to calculate the liquid’s mass. This method provides a reliable way to determine the mass of any liquid.
Understanding Liquid Properties: Unraveling the Secrets of Density
In the realm of science, liquids occupy a fascinating world of their own. To comprehend the behavior and characteristics of liquids, we must delve into the fundamental concept of density. Simply put, density refers to the mass of a substance per unit volume. This intricate relationship between mass, volume, and density unlocks the doors to understanding the properties of liquids.
Consider a scenario where you wish to determine the mass of a liquid in a beaker. Without knowing the liquid’s density, this task would be akin to navigating a maze without a map. However, armed with this crucial piece of information, we can effortlessly calculate the liquid’s mass using the formula: Mass = Density × Volume.
Now, let’s unravel the intricate relationship between these three fundamental concepts:
- Mass: The mass of a substance refers to the amount of matter it contains.
- Volume: Volume measures the amount of space an object or substance occupies in three-dimensional space.
- Density: Density intertwines mass and volume, providing a measure of how tightly packed the matter is within a substance.
By understanding these concepts and their interconnectedness, we gain a deeper comprehension of the behavior and characteristics of liquids, enabling us to unravel the secrets of this captivating realm of science.
Measuring Liquid Volume: Precision and Accuracy
When working with liquids, precise and accurate volume measurements are crucial. Whether you’re a scientist conducting experiments, a chef following a recipe, or simply mixing paints, knowing how much liquid you have is essential. Let’s delve into the common methods for measuring liquid volume and why it matters so much.
Graduated Cylinders: Precision at Your Fingertips
Graduated cylinders are transparent tubes with etched markings that indicate volume. They come in various sizes, from small (5 mL) to large (1000 mL or more). The markings allow for precise readings, making graduated cylinders ideal for measuring small volumes of liquids with high accuracy.
Beakers: Volume Estimation in Larger Quantities
Beakers are cup-shaped containers with a spout for pouring. While they are not as precise as graduated cylinders, beakers are suitable for measuring larger volumes of liquids. Their wide mouths allow for easy pouring and mixing, making them a versatile tool for many applications.
Importance of Accurate Volume Measurements
Accuracy in volume measurements is paramount. In science, precise volumes are crucial for:
- Determining chemical concentrations
- Performing quantitative analyses
- Reproducing experiments
In cooking, accuracy ensures:
- Proper balance of ingredients
- Consistent results
- Optimal flavor
In any field, accurate volume measurements help you avoid errors, ensure safety, and achieve desired outcomes.
Tips for Precise Volume Measurements
- Use the correct equipment for the volume range you need.
- Hold the graduated cylinder or beaker vertically and at eye level to read the markings accurately.
- Estimate the volume to the nearest scale division.
- Double-check your measurements to minimize errors.
Determining Liquid Density: Unlocking the Secrets of Liquid Properties
Every liquid possesses a unique identity defined by its density, a critical parameter that unlocks essential insights into its behavior and composition. Understanding liquid density empowers us to unravel the mysteries of the liquid world.
Delving into the Treasure Trove of Liquid Density
The quest for liquid density values can lead you to tables and databases, veritable storehouses of knowledge containing a vast array of data on liquids. These resources hold the key to discovering the appropriate density value for the liquid in question, a crucial step in unraveling its true nature.
Tips for Navigating the Density Landscape
Selecting the correct density value is akin to searching for a needle in a haystack. Several factors come into play, including temperature and pressure, which can subtly alter the liquid’s density. By carefully considering these variables, you can pinpoint the most accurate density value, ensuring that your subsequent calculations are built on a solid foundation.
Mastering the Art of Liquid Density Determination
With the appropriate density value in hand, you can embark on the journey of calculating liquid mass. Begin by delving into the formula that governs this relationship:
Mass = Density × Volume
This equation forms the cornerstone of liquid mass determination, a powerful tool that empowers you to unravel the mysteries of the liquid’s composition.
Calculating Liquid Mass: A Comprehensive Guide
In the realm of chemistry and physics, understanding the properties of liquids is crucial. Key among them is density, which plays a pivotal role in determining the mass of a liquid. This guide will delve into the intricacies of calculating liquid mass, equipping you with the knowledge and steps to navigate this scientific endeavor.
Formula for Calculating Mass
The formula for calculating the mass of a liquid is:
Mass = Density × Volume
Where:
- Mass is the mass of the liquid in grams (g)
- Density is the density of the liquid in grams per cubic centimeter (g/cm³)
- Volume is the volume of the liquid in cubic centimeters (cm³)
Steps Involved in Calculating Mass
To determine the mass of a liquid, follow these steps:
- Measure the volume of the liquid: Use a graduated cylinder or beaker to accurately measure the volume of the liquid. The volume is expressed in cubic centimeters (cm³).
- Obtain the density of the liquid: Refer to tables or databases to find the density of the liquid. The density is typically given in grams per cubic centimeter (g/cm³).
- Multiply density by volume: Apply the formula, Mass = Density × Volume, by multiplying the density value by the volume measurement obtained in step 1. The result is the mass of the liquid in grams (g).
Example Calculation
Let’s consider an example to illustrate the process of calculating liquid mass. Suppose we have 25 cm³ of water. The density of water at room temperature is 1 g/cm³.
Step 1: Measure volume
Volume = 25 cm³
Step 2: Obtain density
Density = 1 g/cm³
Step 3: Multiply density by volume
Mass = Density × Volume
Mass = 1 g/cm³ × 25 cm³
Mass = 25 grams
Therefore, the mass of 25 cm³ of water is 25 grams.
Example Calculation: Unveiling the Mass of Liquids
To solidify our understanding of liquid mass calculation, let’s embark on a practical example:
Consider a graduated cylinder brimming with 100 milliliters (mL) of water*. Water, as you may know, has a tabulated density of **1 gram per milliliter (g/mL).
Using the formula Mass = Density × Volume, we can determine the mass of the water:
Mass = Density × Volume
Mass = 1 g/mL × 100 mL
Mass = **100 grams**
This calculation reveals that the 100 mL of water possesses a mass of 100 grams.
Remember:
- Accurate measurements are crucial for reliable results.
- Always use the appropriate units in calculations.
- For instance, if the volume had been given in liters (L), we would have converted it to mL (1 L = 1000 mL) before performing the calculation.
By following these steps, you can confidently tackle any liquid mass calculation, empowering you to unravel the mysteries of liquids in your daily life and scientific endeavors.