Understanding Lewis Structures: Visualizing Electron Configuration And Chemical Behavior
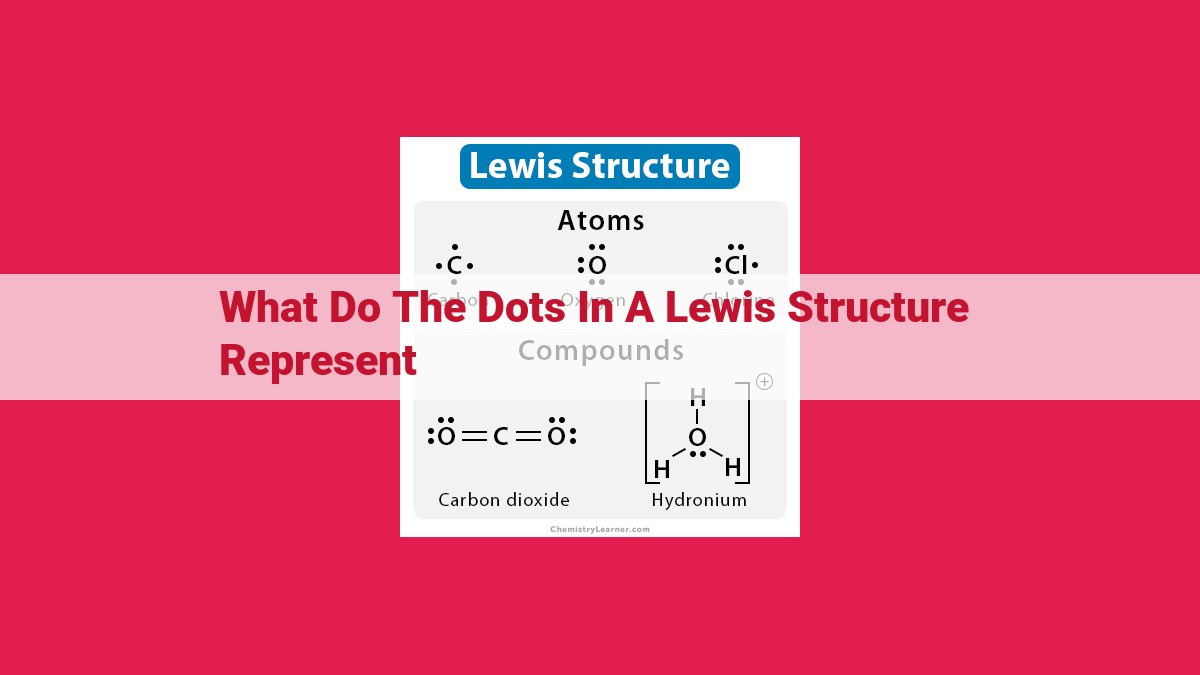
In Lewis structures, dots represent valence electrons that are not involved in chemical bonding. These dots can indicate lone pairs, which are electrons that are not shared with any other atom, or bonding pairs, which are electrons that are shared between two atoms. Lone pairs are usually represented by two dots, while bonding pairs are represented by a single line. The dots help to visualize the electronic configuration of atoms and molecules, allowing chemists to predict their chemical behavior and properties.
Understanding Lewis Structures: The Language of Chemistry
Imagine chemistry as a lively conversation between atoms, communicating their intentions through a visual language called Lewis structures. These structures are like blueprints that reveal the arrangements of electrons within molecules, providing us with crucial insights into their behavior.
A Lewis structure is a symbolic representation of an atom or molecule that shows how many electrons are shared or occupied by each atom. It gives us a clear picture of the molecule’s electronic structure, enabling us to understand, predict, and analyze chemical reactions.
The Significance of Dots: Unraveling the Language of Lewis Structures
In the realm of chemistry, Lewis structures emerge as a powerful tool to visualize the electronic arrangement and bonding patterns of molecules. At the heart of these structures lie tiny dots, each carrying immense significance in deciphering the molecular architecture.
Lone Pairs: Dots on Their Own
Lone pairs are electrons that belong exclusively to a single atom within a molecule. These maverick electrons are not actively involved in bonding and prefer to occupy their own space. In a Lewis structure, lone pairs are represented by two dots placed beside the atom they are associated with. For instance, in the water molecule (H2O), each hydrogen atom proudly displays two dots representing its two lone electrons, while the oxygen atom has four dots signifying its two lone pairs.
Bonding Pairs: Dots Dancing Together
Bonding pairs, on the other hand, are electrons that connect atoms and form the foundation of chemical bonds. In a Lewis structure, bonding pairs are represented by lines, each line symbolizing two shared electrons. These lines connect the participating atoms, highlighting their interdependence. In the methane molecule (CH4), four lines radiate from the carbon atom, each connecting it to a hydrogen atom. These lines represent the four bonding pairs that hold this molecule together.
Dots and Electronegativity: A Delicate Balance
The arrangement of dots in a Lewis structure is influenced by the electronegativity of the atoms involved. Electronegativity measures an atom’s attraction for electrons. Atoms with higher electronegativity tend to pull electron pairs closer to themselves, leaving fewer dots on the neighboring atoms. For example, in the hydrogen chloride molecule (HCl), the chlorine atom is more electronegative than hydrogen, so the bonding pair is shifted towards the chlorine atom.
The dots in a Lewis structure are more than mere placeholders. They provide crucial information about the electronic distribution within a molecule, shedding light on the nature of lone pairs, bonding pairs, and the interplay of electronegativity. By unraveling the language of dots, we gain a deeper understanding of the invisible forces that govern the structure and behavior of matter.
Lone Pairs: Electrons on the Go
- Describe the characteristics and location of lone pairs, including their association with electronegative atoms.
Lone Pairs: Electrons on a Solo Adventure
In the intricate realm of chemistry, we encounter a fascinating group of electrons known as lone pairs. Imagine a group of electrons left unattached, like solitary wanderers in a vast chemical landscape. These lone pairs possess unique characteristics that influence the behavior and properties of molecules.
Where to Find Lone Pairs
Lone pairs are typically found around electronegative atoms, which have a strong pull on electrons. Electronegative atoms, like oxygen, nitrogen, and fluorine, prefer to hold onto their electrons tightly, leaving some electrons unshared and forming lone pairs.
Characteristics of Lone Pairs
Lone pairs are non-bonding electrons, meaning they are not involved in any chemical bonds. They typically occupy the outermost electron shell of an atom, orbiting in specific orbitals known as p or d orbitals. These orbitals have a distinct shape that creates a region of high electron density around the atom.
The Impact of Lone Pairs
Lone pairs have a significant impact on the electronic structure and properties of molecules. They:
- Repel other electrons: Lone pairs occupy space in the molecule’s electron cloud, creating areas of high electron density. This repulsion can affect the arrangement of atoms and the formation of chemical bonds.
- Influence molecular shape: The presence of lone pairs can influence the geometry of molecules, as they can distort the shape of the electron cloud around the atom.
- Can participate in reactions: While lone pairs are typically non-reactive, they can occasionally participate in chemical reactions, forming new bonds or donating electrons.
Examples of Lone Pairs
To illustrate the concept of lone pairs, let’s consider a molecule of water (H2O). The oxygen atom in water has two lone pairs of electrons, which occupy p-orbitals perpendicular to the bonding orbitals. These lone pairs contribute to the bent shape of the water molecule and its polarity.
Bonding Pairs: Sharing is Caring – A Journey into Covalent Bonds
In the realm of atomic interactions, where electrons dance and bonds form, lies a fascinating concept known as bonding pairs. These are the electrons that hold atoms together, forging the intricate web of molecules that shape our world.
** Bonding pairs** are formed when atoms share electrons, creating a special kind of bond called a covalent bond. Covalent bonds are formed between atoms with similar electronegativities, meaning they have a comparable desire for electrons. When two such atoms come together, they each contribute one electron to the bond, resulting in a shared pair of electrons that belong to both atoms.
These shared electrons form a molecular orbital, a region of space where the electrons are most likely to be found. The overlap of atomic orbitals creates the molecular orbital, providing a home for the bonding pair. The electrons in the bonding pair are constantly moving within this molecular orbital, creating a dynamic and stable bond between the atoms.
The number of bonding pairs between atoms determines the bond order, which indicates the strength of the bond. A single bond consists of one bonding pair, a double bond has two bonding pairs, and a triple bond has three bonding pairs. The more bonding pairs, the stronger the bond between the atoms.
Understanding bonding pairs is crucial for unraveling the mysteries of chemical interactions, from simple molecules to complex biomolecules. It helps us comprehend how atoms interact to form the substances that make up our universe. By grasping the concept of bonding pairs, we embark on a journey into the fascinating world of covalent bonding and the fundamental forces that shape the molecular tapestry of our existence.
Related Concepts: A Deeper Dive
In our exploration of Lewis structures, we’ve touched upon several fundamental concepts that further illuminate the significance of these diagrams. Let’s delve into these concepts, unraveling their intricate relationships with Lewis structures.
Electronegativity: The Attraction Game
Electronegativity measures an atom’s affinity for electrons. The more electronegative an atom, the stronger its pull on the shared electrons in a covalent bond. Understanding electronegativity is crucial in predicting bond polarity and the likelihood of electron transfer. Lewis structures ingeniously indicate electronegativity differences, guiding our understanding of molecular interactions.
Covalent Bonds: Sharing the Electron Love
Covalent bonds form when atoms share electron pairs, creating a stable molecular structure. Through Lewis structures, we can visualize these bond connections and explore their effects on molecular geometry. Covalent bonding is the backbone of countless organic and inorganic compounds, shaping the vast tapestry of our chemical world.
Electronic Structure: The Foundation of Chemistry
The electronic structure of an atom refers to the arrangement of its electrons in specific energy levels. This arrangement influences an atom’s chemical properties, including its electronegativity and bonding tendencies. Lewis structures are a graphical representation of electronic structure, providing insights into the stability and reactivity of molecules.