Krypton: Atomic Structure And Neutron Count For Diverse Applications In Noble Gas Research
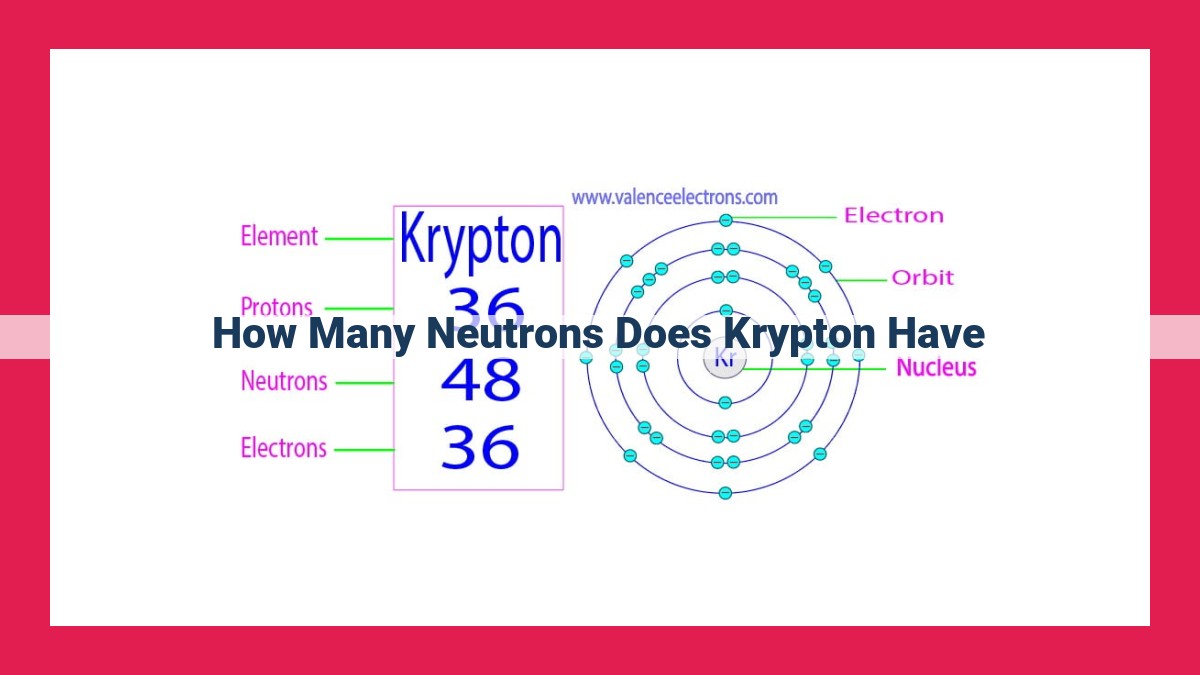
Krypton has 48 neutrons. As a noble gas, understanding its atomic structure is crucial for its applications. Krypton’s atomic number (36) determines the number of protons, while its mass number varies due to isotopic composition. The stable isotopes of krypton have varying neutron-to-proton ratios, which influence their stability. By considering the weighted average of these isotopes, the average number of neutrons in natural krypton is calculated to be 48. This neutron count affects the isotope’s reactivity and stability, influencing krypton’s various applications.
- Introduce krypton as a noble gas and its applications.
- Highlight the importance of understanding its atomic structure.
Krypton: Unveiling the Secrets of its Atomic Structure
Krypton, a noble gas with a distinctive atomic structure, plays a crucial role in various applications from lighting to medical imaging. To delve into the world of krypton, it’s essential to understand the building blocks of its atom, starting with its unique atomic composition.
Atomic Number and Proton Number: The Foundation of Krypton’s Identity
Each element is characterized by its atomic number, which represents the number of protons within its nucleus. Krypton bears an atomic number of 36, indicating the presence of 36 protons at its core. This fundamental property defines krypton’s position in the periodic table and governs its chemical behavior.
Mass Number and Nucleon Number: Exploring Krypton’s Isotopic Variation
Beyond the atomic nucleus, krypton’s atomic structure is further shaped by its mass number and nucleon number. The mass number denotes the total number of protons and neutrons combined, while the nucleon number refers to the total number of protons and neutrons in the nucleus. Krypton’s mass number can vary due to the existence of isotopes, atoms with the same atomic number but different mass numbers. This variation arises from varying numbers of neutrons in the nucleus.
Unveiling Krypton’s Atomic Structure: The Mystery of Proton and Neutron Numbers
Krypton, a noble gas renowned for its inertness, plays a crucial role in various fields, from lighting to medical imaging. To fully comprehend its properties and applications, we must delve into its atomic structure, particularly the number of protons and neutrons within its nucleus.
Atomic Number: The Identity Key
Every atom is characterized by its atomic number, which equals the number of protons in its nucleus. For krypton, this number is 36. Protons, positively charged particles, determine an element’s identity. They define the element’s position on the periodic table and influence its chemical behavior.
Proton Number: The Atomic Fingerprint
The atomic number is also known as the proton number, as it directly correlates to the number of protons within the atom. For krypton, its proton number is 36, which means that every krypton atom contains 36 protons. This unique proton count distinguishes krypton from all other elements and gives it its distinct properties.
Mass Number and Nucleon Number: Unraveling the Structure of Krypton’s Atomic Core
At the heart of every atom lies a nucleus, a tiny but mighty powerhouse that holds the key to an element’s unique properties. For krypton, a noble gas with a wide array of applications, understanding the intricacies of its atomic core is crucial. Two important concepts that shed light on krypton’s nuclear structure are mass number and nucleon number.
Mass number, often represented by the symbol A, is the total number of protons and neutrons found within an atom’s nucleus. Nucleon number, on the other hand, refers exclusively to the sum of protons and neutrons, excluding electrons. Protons, with their positive charge, balance the negative charge of electrons, while neutrons contribute to the atom’s overall mass without carrying a charge.
Krypton’s atomic number, denoted by Z, is 36, indicating that every krypton atom possesses 36 protons. However, krypton’s mass number can vary due to the presence of different isotopes. Isotopes are atoms of the same element that share the same number of protons but differ in the number of neutrons. For example, the most common isotope of krypton, krypton-84, has a mass number of 84, indicating that its nucleus contains 36 protons and 48 neutrons (84 – 36 = 48). Other isotopes of krypton, such as krypton-82 and krypton-86, have mass numbers of 82 and 86, respectively, corresponding to different neutron counts.
These variations in mass number arise from the varying neutron-to-proton ratios in krypton’s isotopes. Stable isotopes, like krypton-84, have neutron-to-proton ratios that favor stability, while unstable isotopes tend to have imbalanced ratios. The neutron-to-proton ratio influences an isotope’s stability because neutrons provide stability to the nucleus by counteracting the repulsive forces between positively charged protons.
**Krypton’s Isotopic Symphony: Unraveling the Neutron-Proton Dance**
Neutron-Proton Ratio: The Balancing Act of Isotope Stability
The neutron-to-proton ratio plays a pivotal role in determining the stability of an atomic nucleus. Stable isotopes possess a balanced ratio, allowing for a harmonious coexistence of neutrons and protons. Unstable isotopes, on the other hand, exhibit an imbalance in this ratio, leading to radioactive decay.
Krypton’s Stable Isotope Ensemble
Krypton, a noble gas residing in the atmospheric tapestry, boasts six stable isotopes: krypton-78, krypton-80, krypton-82, krypton-83, krypton-84, and krypton-86. Each isotope dances to a unique neutron-to-proton rhythm, maintaining a harmonious balance that ensures their stability.
Unveiling the Neutron-Proton Ratios
Investigating the neutron-to-proton ratios of krypton’s stable isotopes, we uncover a fascinating pattern. The lighter isotopes, such as krypton-78, possess a lower ratio, while the heavier isotopes, like krypton-86, exhibit a higher ratio. This variation in neutron-to-proton ratios contributes to the distinctive properties of each isotope.
Implications for Applications
Krypton’s diverse isotopic composition finds practical applications in various fields. For instance, krypton-85, with its unique neutron-to-proton ratio, serves as a versatile radioactive tracer in medical diagnostics. Additionally, krypton-86, featuring a higher neutron count, finds employment in nuclear energy as a moderator to regulate neutron energies.
Delving into Krypton’s Atomic Structure: Unveiling the Number of Neutrons
Krypton, a noble gas renowned for its versatility, holds within its atomic structure the key to its extraordinary properties. Understanding the number of neutrons in krypton is crucial for unraveling its isotopic composition and diverse applications.
Determining Neutron Count Based on Isotopic Composition
Krypton exists in various forms known as isotopes, each with a distinct atomic mass due to varying neutron numbers. To determine the number of neutrons in a particular isotope, we subtract the atomic number (number of protons) from the mass number.
Calculating the Neutron Count of Natural Krypton
Natural krypton is a mixture of isotopes, and its overall neutron count represents the average of its isotopic composition. The most abundant isotope, krypton-84, has an atomic number of 36 and a mass number of 84. Using our formula, we can calculate the neutron count:
Neutron count = Mass number - Atomic number
Neutron count = 84 - 36
Neutron count = **48**
Therefore, each atom of natural krypton contains 48 neutrons.
Implications of Neutron Count
The neutron count significantly influences isotope stability and reactivity. Stable isotopes have a balanced neutron-to-proton ratio, contributing to their longevity. Krypton-84, with its 48 neutrons, has a stable neutron-to-proton ratio of 1.33.
Neutron count also affects krypton’s applications. For example, krypton-85, with an unstable neutron-to-proton ratio, is used in medical imaging, while heavier isotopes like krypton-86 are utilized in nuclear medicine.
In conclusion, understanding the number of neutrons in krypton is essential for comprehending its isotopic composition and versatility. Natural krypton’s 48 neutrons contribute to its stability and pave the way for its diverse applications in science and medicine.
The Enigmatic Krypton: Delving into the Significance of Neutron Count
Krypton, an enigmatic noble gas, holds a pivotal position in the realm of chemistry and physics. Understanding its atomic structure, particularly the neutron count, is paramount to comprehending its unique properties and diverse applications.
Neutron Count and Isotope Stability
The neutron count of an atom, coupled with its proton count, determines its isotopic composition. Isotopes, atoms with the same atomic number but varying neutron numbers, exhibit distinct stability profiles. In the case of krypton, the neutron-to-proton ratio plays a crucial role in determining isotope stability.
Stable isotopes possess a balanced neutron-to-proton ratio, which ensures their resilience against radioactive decay. Conversely, unstable isotopes, characterized by an imbalance in this ratio, undergo decay processes to achieve stability. Krypton’s stable isotopes, krypton-82, krypton-83, krypton-84, and krypton-86, showcase neutron-to-proton ratios that promote longevity.
Neutron Count and Krypton’s Applications
The neutron count has far-reaching implications for krypton’s reactivity and its subsequent applications. Isotopes with higher neutron counts exhibit reduced reactivity, rendering them more suitable for specific applications. For instance, krypton-85, a radioactive isotope with a high neutron count, is employed in medical imaging and radiation therapy.
In incandescent lighting, krypton-86, with its low reactivity, serves as an effective fill gas, contributing to extended bulb life and improved light quality. The stability of krypton-86 also makes it invaluable in high-efficiency laser systems.
Krypton’s neutron count, interwoven with its atomic structure, forms the foundation for its remarkable properties and diverse applications. Unraveling the relationship between neutron count and isotope stability reveals the essence of this enigmatic element. Understanding these intricacies enables us to harness krypton’s potential, ranging from advanced medical technologies to innovative lighting solutions.