Key Differences Between Chemical And Nuclear Reactions: Energy Release, Matter Transformations, And Elemental Changes
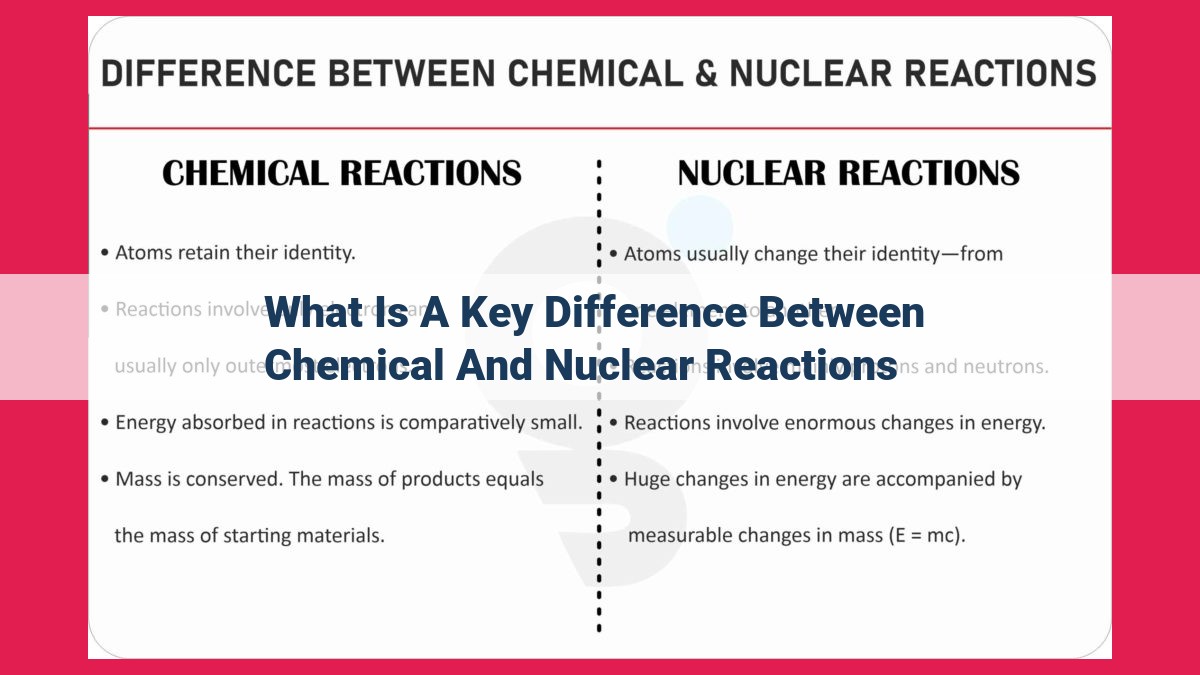
A key difference between chemical and nuclear reactions lies in the energy and matter transformations involved. While chemical reactions involve a rearrangement of electrons in chemical bonds, releasing or absorbing smaller amounts of energy, nuclear reactions focus on changes in atomic nuclei, releasing or absorbing significantly greater amounts of energy. Chemical reactions conserve the number of atoms of each element, but nuclear reactions can result in the creation or destruction of atoms, leading to the formation of new elements.
Energy Transformations in Chemical and Nuclear Reactions
In the realm of chemistry, energy plays a pivotal role, driving the transformations of matter and unleashing its hidden potential. Energy is the capacity to do work or produce a change, and in chemical and nuclear reactions, it undergoes fascinating transformations.
Thermodynamics: A Guiding Principle
Thermodynamics provides a framework for understanding these energy changes. It dictates the flow and transformation of energy within systems and surroundings. One key concept is enthalpy (H), a measure of the total energy within a system, including its internal energy and pressure-volume work.
Enthalpy and Heat of Reaction
In chemical reactions, enthalpy change (ΔH) measures the energy absorbed or released during the reaction. When ΔH is positive, the reaction is endothermic and absorbs energy from its surroundings. Conversely, negative ΔH reactions are exothermic, releasing energy into the surroundings.
Nuclear Reactions: A Paradigm Shift
Nuclear reactions, unlike their chemical counterparts, involve the rearrangement of atomic nuclei, releasing immense amounts of energy. These reactions can alter the very composition of matter, forming new elements. The energy released in nuclear reactions, such as in nuclear power plants and nuclear weapons, far exceeds that of chemical reactions.
Comparing Chemical and Nuclear Reactions
While both chemical and nuclear reactions involve energy transformations, they differ significantly in their mechanisms and consequences. Chemical reactions primarily involve the rearrangement of electrons within atoms and molecules, while nuclear reactions delve into the realm of atomic nuclei, leading to potentially explosive outcomes.
Rearranging Matter: Reactants and Products
In the intricate dance of chemical and nuclear reactions, reactants transform into products, rearranging the building blocks of matter. Chemical equations, like recipes for molecular transformations, provide a roadmap for these changes. Stoichiometry, the study of quantitative relationships between reactants and products, ensures that the ingredients are balanced for a successful outcome.
Nuclear reactions, unlike their chemical counterparts, stand out as alchemists of a different realm. They possess the remarkable ability to create entirely new elements. When atomic nuclei collide, they can fuse, split, and even rearrange their protons and neutrons, giving birth to elements that never existed before. This extraordinary power highlights the profound differences between chemical and nuclear reactions, delving into the very fabric of matter itself.
The Role of Bonds in Chemical and Nuclear Reactions
In the realm of chemistry, the formation and breaking of bonds are the driving forces behind every reaction. From the simplest of chemical interactions to the explosive power of nuclear transformations, bonds play a central role in shaping the molecular world around us.
Chemical Bonds: The Glue of Molecules
In chemical reactions, molecules interact through various types of bonds, each with its own unique characteristics. Covalent bonds form when atoms share electrons, creating a strong attraction that holds them together. Ionic bonds, on the other hand, result from the transfer of electrons between atoms, resulting in the formation of oppositely charged ions that attract each other. Weaker bonds, such as hydrogen bonds and van der Waals forces, also contribute to the structure and behavior of molecules.
Nuclear Bonds: The Power Within
Nuclear reactions involve interactions at the subatomic level, where the fundamental forces of nature come into play. The most dominant force in the nucleus is the strong nuclear force, which binds protons and neutrons together despite their mutual repulsion from electrostatic forces. This force is responsible for the stability of atomic nuclei.
When nuclei undergo reactions, such as fission or fusion, the rearrangement of protons and neutrons leads to changes in the nuclear forces. Fission involves the splitting of a heavy nucleus, releasing vast amounts of energy. Conversely, fusion combines lighter nuclei into heavier ones, also releasing energy. Both processes involve alterations in the binding energy of the nuclei, which is related to the strength of the nuclear forces holding them together.
Understanding the role of bonds in both chemical and nuclear reactions provides a deeper insight into the transformative processes that occur in our world. From the subtle changes in molecular structure during chemical reactions to the explosive power of nuclear reactions, bonds are the fundamental forces that shape and control the chemical and nuclear landscape.
Factors Influencing Reaction Rates: A Tale of Chemical and Nuclear Dance
In the realm of chemistry and nuclear reactions, the pace at which reactants transform into products is not a matter of chance but is subject to various factors that influence their reaction rates. Join us as we dive into the fascinating world of reaction rates, contrasting the influence of concentration, temperature, and other elements on chemical and nuclear reactions.
The Power of Concentration: A Crowded Chemical Dance
Imagine a bustling dance floor where reactants are represented by a crowd of dancers. As the concentration of dancers increases, the likelihood of them colliding and interacting with each other soars. This increased proximity fuels a higher reaction rate as more dance partners find each other. However, in the world of nuclear reactions, concentration plays a less significant role, as these reactions are driven by the strong nuclear force, which is independent of the number of reactants present.
The Heat of the Moment: Temperature’s Impact
Temperature, like a fiery salsa beat, ignites the passion of reactions. As the temperature rises, reactant molecules gain more kinetic energy and move faster, increasing the frequency of their collisions. Consequently, reaction rates skyrocket. In contrast, nuclear reactions are largely unaffected by temperature, as the nuclear force is so strong that it dominates over thermal energy.
Activation Energy: A Dance with a Hidden Barrier
Imagine a dance where dancers must overcome a small hurdle, represented by activation energy. This hurdle determines the minimum amount of energy required for a reaction to occur. In chemical reactions, catalysts, like dance instructors, can lower the activation energy, making it easier for reactants to overcome this hurdle and waltz into products. Nuclear reactions, on the other hand, possess a much higher activation energy, making them less susceptible to catalysis.