How To Calculate Kb From Ph: A Step-By-Step Guide For Maximizing Acid-Base Understanding
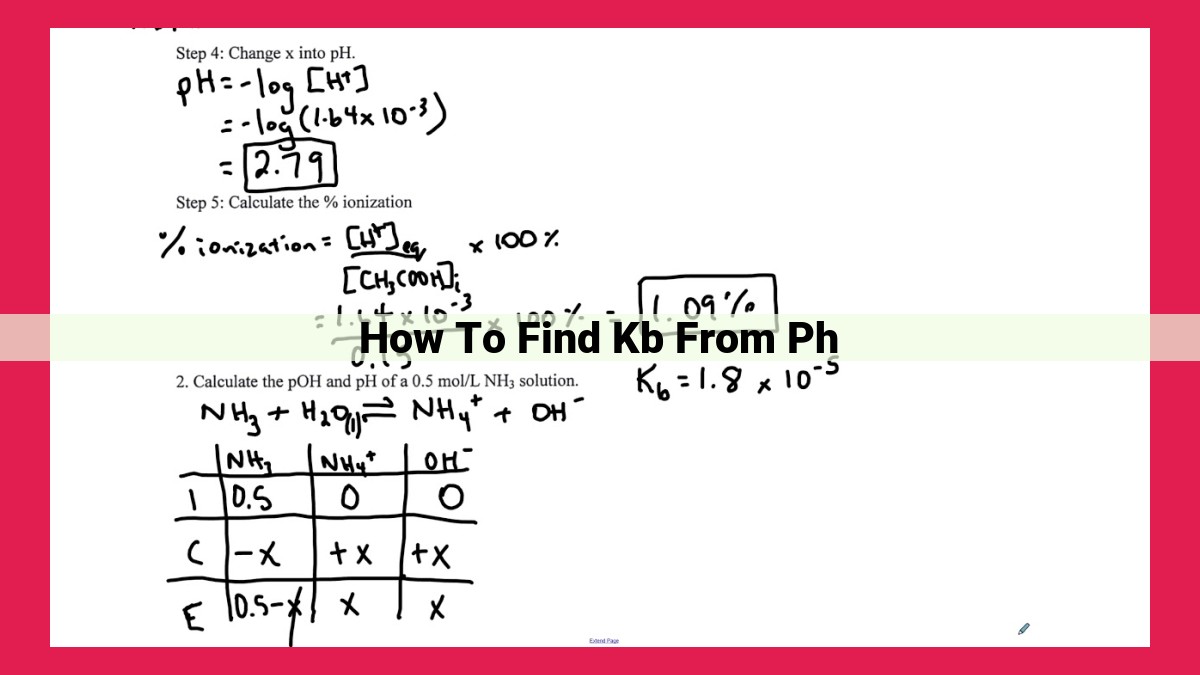
To find Kb from pH, follow these steps:
- Measure or obtain the pH of the solution.
- Determine the pKa of the weak acid using a reference table or calculation.
- Calculate Kb using the Henderson-Hasselbalch equation: Kb = 10^(-14) / Ka, where Ka is the acid dissociation constant.
Understanding the Significance of pH and Acid-Base Equilibrium
pH: A Measure of Acidity and Alkalinity
- What is pH? It stands for -potential of hydrogen-, and is the measure of acidity or alkalinity of a solution. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
- Why is pH important? It plays a critical role in a wide range of scientific and industrial applications, such as determining the effectiveness of cleaning solutions, the stability of food products, and the health of biological systems.
Acid-Base Equilibrium: The Balancing Act
- Acid-base equilibrium refers to the reversible chemical reaction between an acid and a base. When an acid dissolves in water, it releases hydrogen ions (H+), making the solution acidic. Conversely, when a base dissolves in water, it releases hydroxide ions (OH-), making the solution basic.
- pH measurement is closely related to acid-base equilibrium. The pH of a solution indicates the relative concentration of hydrogen ions and hydroxide ions present.
pH Measurement: Definitions and Concepts
When it comes to understanding the world around us, both in the scientific realm and in our everyday lives, pH plays a vital role. It’s a measure of how acidic or alkaline a substance is, and it has a profound impact on everything from chemical reactions to the health of our bodies.
In this section, we’ll dive into the fascinating world of pH measurement, exploring its key concepts and their significance. We’ll start by defining pH and explaining why it matters.
Defining pH: A Measure of Acidity and Alkalinity
pH stands for potential of hydrogen, and it’s a measure of the concentration of hydrogen ions (H+) in a solution. The pH scale ranges from 0 to 14, with 7 representing neutrality. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are alkaline or basic.
pH is a critical parameter in many scientific and industrial applications. For example, it affects the rate of chemical reactions, the stability of compounds, and the solubility of substances. In biology, pH plays a crucial role in maintaining the delicate balance of life processes. The pH of blood, for instance, must be tightly controlled within a narrow range to ensure proper bodily functions.
Temperature and pH: A Dynamic Relationship
It’s important to note that pH is not a fixed property of a solution. It can change with temperature. As temperature increases, the pH of a solution tends to decrease, meaning it becomes more acidic. This is because the higher energy of the molecules at higher temperatures disrupts the equilibrium between hydrogen ions and other ions in the solution.
Acid Dissociation Constant: Quantifying Acidity
The acid dissociation constant (Ka) is a measure of the strength of an acid. It represents the tendency of an acid to donate a hydrogen ion. The higher the Ka, the stronger the acid. Ka is related to pH by the following equation:
pH = -log[H+]
where [H+] is the concentration of hydrogen ions.
Dissociation Equilibrium: Balancing the Ions
When an acid dissolves in water, it undergoes dissociation, releasing hydrogen ions and other ions into the solution. This process is reversible, with hydrogen ions recombining to form the acid. The equilibrium between the acid and its ions is determined by the Ka.
Understanding these key concepts of pH measurement is essential for accurate pH determination and for comprehending the behavior of acids and bases in various chemical and biological systems.
Acid Dissociation Constants (Ka and Kb)
- Define Ka and Kb and explain the difference between them.
- Explain the relationship between Ka and Kb and pH.
- Introduce related concepts: pKa, pKb, and the Henderson-Hasselbalch equation.
Acid Dissociation Constants: Unveiling the Secrets of Acidity and Basicity
In the realm of chemistry, acidity and basicity hold crucial significance. To dissect these concepts, we must embark on a journey into the world of acid dissociation constants, symbolized by Ka and Kb.
Defining Ka and Kb
Ka (acid dissociation constant) quantifies the tendency of an acid to lose a proton, while Kb (base dissociation constant) measures the propensity of a base to gain a proton.
The Interplay of Ka and Kb
These constants exhibit an inverse relationship: Ka x Kb = Kw, where Kw is the ionic product of water at a specific temperature. This interplay reveals that a strong acid with a large Ka will have a correspondingly small Kb, and vice versa.
Introducing pKa, pKb, and the Henderson-Hasselbalch Equation
To facilitate comparisons of acid-base strengths, scientists introduced the concepts of pKa and pKb. These negative logarithmic values provide a convenient way to express Ka and Kb, respectively.
The Henderson-Hasselbalch equation establishes a critical connection between pH, pKa, and pKb: pH = pKa + log([A-]/[HA]), where [A-] represents the concentration of the conjugate base and [HA] represents the concentration of the acid.
Finding Kb from pH: A Step-by-Step Guide
Are you grappling with the challenge of calculating the base dissociation constant (Kb) from a given pH value? Fret not! This comprehensive guide will unravel the intricacies of this calculation using the Henderson-Hasselbalch equation.
Step 1: Understanding the Constants
The Henderson-Hasselbalch equation is a powerful tool that relates pH to Kb. It is written as:
pH = pKb + log([A-]/[HA])
where:
- pH is the acidity or alkalinity of the solution.
- pKb is the negative logarithm of Kb.
- [A-] is the concentration of the conjugate base.
- [HA] is the concentration of the weak acid.
Step 2: Rearranging the Equation
To calculate Kb from pH, we need to rearrange the Henderson-Hasselbalch equation:
Kb = **10^(-(pH – pKb))**
Step 3: Calculating pKb
pKb is often a known value or can be calculated from the acid dissociation constant (Ka) of the conjugate acid using the relationship:
pKb + pKa = 14
Step 4: Plugging In the Values
Once you have the values of pH and pKb, simply substitute them into the rearranged Henderson-Hasselbalch equation to calculate Kb:
Kb = **10^(-(pH – pKb))**
Worked Example
Let’s say you have a solution with a pH of 8.0 and a pKb of 6.0. To find Kb:
Kb = 10^(-(8.0 – 6.0))
= 10^(-2)
= 0.01
Therefore, the base dissociation constant (Kb) of the solution is 0.01.
Related Concepts and Their Significance
In the realm of pH measurement and acid-base equilibrium, several concepts play crucial roles in understanding and calculating Kb (base dissociation constant). These concepts not only complement our knowledge of Kb but also provide valuable insights into the behavior of acids and bases.
Temperature and Kb Calculations
Temperature exerts a profound influence on Kb calculations. As temperature rises, the Kb values of acids and bases generally increase. This is because higher temperatures favor the dissociation of acids and bases, leading to a higher concentration of ions and a corresponding increase in Kb. Therefore, it is essential to consider the temperature at which Kb measurements are made.
pKa, pKb, and Kb Calculations
pKa and pKb are logarithmic measures of the strength of acids and bases, respectively. pKa and pKb are inversely related to each other, meaning that a strong acid will have a low pKa and a high pKb. These values provide a convenient way to estimate the relative strengths of acids and bases. In Kb calculations, pKa and pKb are used to determine the relationship between the concentration of the conjugate acid and the Kb of the base.
Henderson-Hasselbalch Equation and Kb
The Henderson-Hasselbalch equation is a fundamental equation that relates pH to the ratio of conjugate acid and base concentrations. This equation is also applicable to the relationship between pH and Kb. By using the Henderson-Hasselbalch equation, we can calculate Kb from experimentally measured pH values. This equation provides a practical tool for determining Kb and understanding the acid-base properties of solutions.
Applications of Kb Calculations
In the world of chemistry and biology, understanding the intricacies of Kb calculations is not merely an academic pursuit but a vital tool with far-reaching applications. From the precision of titrations to the delicate balance of biological buffers, the significance of Kb cannot be overstated.
Chemistry’s Reliance on Kb
In the laboratory, Kb calculations serve as the guiding light in various chemical processes, including:
-
Titrations: Kb plays a crucial role in determining the exact concentration of unknown acids or bases through neutralization reactions. By measuring the pH of the solution and leveraging the Henderson-Hasselbalch equation, chemists can calculate Kb and accurately determine the concentration of the analyte.
-
Buffer Preparation: Buffers, solutions that resist pH changes, are essential components in chemical reactions, maintaining stable pH levels. Kb calculations help chemists design and prepare buffers with specific pH values, catering to the needs of various chemical processes.
Kb’s Significance in Biology
Beyond the realm of chemistry, Kb calculations find their place in the intricate world of biology, particularly in the realm of:
- Buffering in Biological Systems: Biological systems rely heavily on buffers to maintain optimal pH levels. Kb calculations aid in understanding the behavior of these buffers, ensuring the delicate balance of pH necessary for cellular processes like enzyme activity and protein stability.
By unraveling the complexities of Kb calculations, scientists can decipher the intricacies of chemical reactions, pave the way for accurate pH measurements, and delve into the depths of biological processes where pH plays a pivotal role. These applications underscore the significance of Kb calculations, making them an indispensable tool in the pursuit of scientific knowledge and advancements.