Identify Isotopes: Methods In Mass Spectrometry, Nmr, And Radioactive Decay
Identifying isotopes involves determining the number of protons (atomic number) and neutrons (mass number). As isotopes share the same atomic number, they are distinguished by their mass numbers. Mass spectrometry measures the masses of ions, allowing for the separation and identification of isotopes based on their mass-to-charge ratios. Nuclear magnetic resonance (NMR) spectroscopy examines the chemical environment of atoms, providing insights into isotope distributions within molecules. Additionally, radioactive isotopes exhibit unique decay rates, which can be used to differentiate them from stable isotopes.
Isotopes: Unraveling the Secrets of the Atomic World
In the realm of chemistry, isotopes stand as captivating and enigmatic entities, holding immense significance across a vast array of scientific disciplines. These atomic variations, with their identical atomic numbers but distinct mass numbers, play a pivotal role in shaping the world around us.
Unveiling the Nature of Isotopes
The atomic number, a unique identifier for each element on the periodic table, represents the number of protons in an atom’s nucleus. Isotopes of the same element share this atomic number, but they differ in their mass number, which is determined by the number of neutrons in the nucleus. This subtle variation in mass gives rise to isotopes, which are essentially different variants of the same element.
The Multifaceted Importance of Isotopes
Isotopes are far from mere scientific curiosities. Their applications extend far beyond the confines of the laboratory, reaching into diverse fields such as:
- Medicine: Radioactive isotopes, like iodine-131, are used in medical imaging and treatment, offering insights into organ function and enabling targeted therapies.
- Archaeology: Isotopes provide invaluable clues about the age and provenance of artifacts, unraveling historical mysteries and illuminating the passage of time.
- Environmental Studies: Stable isotopes, such as carbon-13, serve as tracers to track the movement of pollutants and understand ecosystem dynamics.
By embracing the multifaceted applications of isotopes, we gain a deeper understanding of the world we inhabit, unlocking its mysteries and harnessing its potential for the betterment of society.
Atomic Number: The Defining Feature
In the realm of chemistry, isotopes reign supreme as the building blocks of matter. These fascinating variants of an element share an atomic number, a fundamental characteristic that sets them apart. The atomic number serves as an element’s unique identifier on the periodic table.
Imagine a family of elements, each with its own distinct set of characteristics. The atomic number is like their family name, a shared trait that indicates their common ancestry. It represents the number of protons within the atom’s nucleus—the central core that governs the element’s identity.
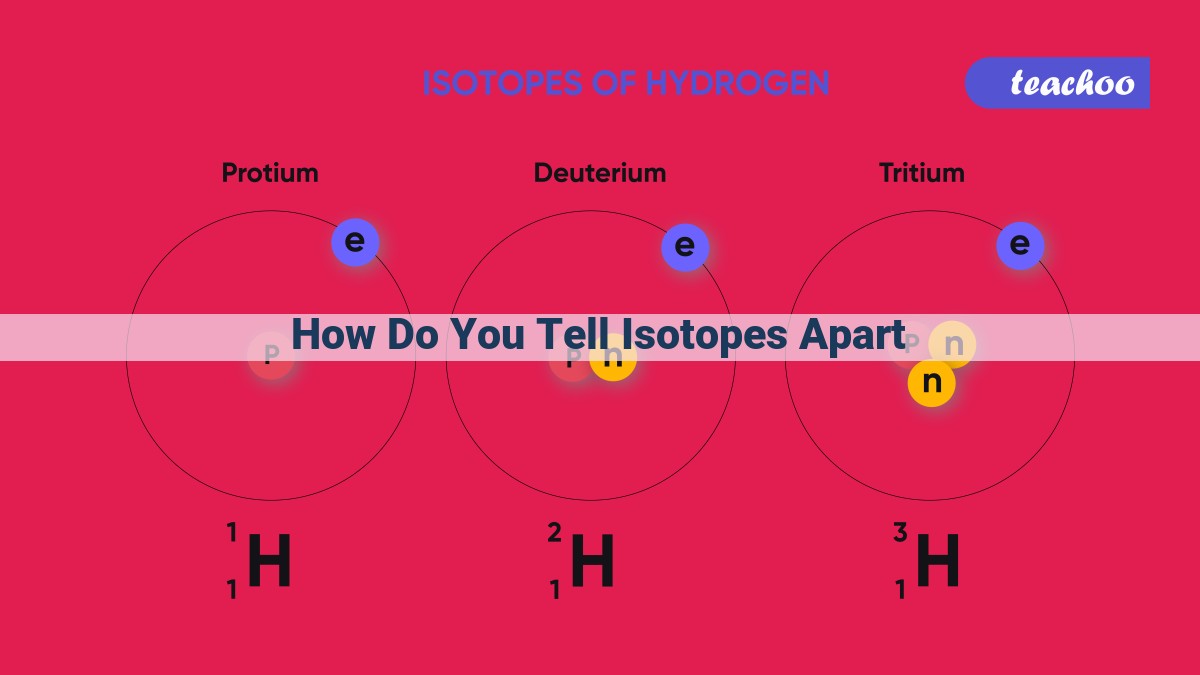
For instance, let’s take a closer look at the hydrogen family. Hydrogen has an atomic number of 1, indicating that every hydrogen atom contains a single proton in its nucleus. This atomic number distinguishes hydrogen from all other elements and places it at the beginning of the periodic table.
Remarkably, isotopes of the same element share the same atomic number. They may have different numbers of neutrons in their nuclei, but their protons remain constant. This means that isotopes have identical chemical properties, as their electronic configurations are determined by the atomic number.
For example, hydrogen has three common isotopes: protium, deuterium, and tritium. While protium is the most abundant and contains only one proton and zero neutrons, deuterium has one proton and one neutron, and tritium has one proton and two neutrons. Despite these variations in mass, all three isotopes share the same atomic number of 1 and belong to the same hydrogen family.
The atomic number serves as a crucial factor in determining the element’s chemical behavior. It governs the number of electrons surrounding the nucleus, which in turn influences the element’s reactivity, bonding capabilities, and place within the periodic table. Understanding the atomic number provides a deeper insight into the fundamental nature of elements and their role in shaping the chemical world around us.
Atomic Mass: Unveiling the Weighted Average of an Element’s Isotopes
In the realm of chemistry, the concept of atomic mass plays a pivotal role in understanding the characteristics of elements. It provides a weighted average of the masses of all the isotopes of an element. Isotopes are variations of an element that share the same number of protons (atomic number) but differ in the number of neutrons.
To grasp the concept of atomic mass, it’s important to visualize a hypothetical scale where each isotope is represented by a small weight. Each weight carries a mass proportional to the abundance of that particular isotope. The atomic mass is then determined by balancing this hypothetical scale, taking into account both the mass and abundance of each isotope.
The masses of isotopes are not uniformly distributed. Some isotopes may be more abundant than others, leading to a skewed weighting in the average. For instance, the element carbon has two stable isotopes: carbon-12 and carbon-14. Carbon-12 is about 99% abundant, while carbon-14 is present in trace amounts. As a result, the atomic mass of carbon is closer to the mass of carbon-12.
This weighted average nature of atomic mass has profound implications in chemistry. When performing calculations involving elemental mass, it’s the atomic mass that is used. This ensures accuracy in determining quantities such as molar mass, which is crucial for stoichiometric relationships and other chemical calculations.
Furthermore, atomic mass provides valuable insights into the behavior of elements in chemical reactions. By comparing the atomic masses of different isotopes, scientists can deduce their relative reactivity and formation mechanisms. This information is essential in fields such as nuclear physics and geochemistry.
In essence, atomic mass is a reflection of an element’s isotopic composition, weighted by abundance. It’s a fundamental property that underpins our understanding of elemental characteristics and plays a critical role in various chemical calculations and applications.
Mass Spectrometry: Unraveling the Secrets of Isotopes
Imagine a detective’s lab where isotopes are the hidden suspects under investigation. Scientists use a powerful tool called mass spectrometry to separate these tiny particles and determine their identities. This technique has played a crucial role in various fields, including medicine, archaeology, and environmental studies.
Mass spectrometry begins with atom ionization. Using a high-energy electron beam or laser, neutral atoms lose electrons, transforming into positively charged ions. These ions are then accelerated through a magnetic field, which acts like a prism, separating them based on their mass and charge.
After acceleration, the ions pass through a detector that measures their mass-to-charge ratio (m/z). This critical information provides scientists with a unique fingerprint for each isotope. By analyzing the abundance of different isotopes, researchers can gather valuable insights about the sample’s origin, age, and Zusammensetzung.
In medicine, mass spectrometry helps identify unknown compounds in biological samples, diagnose genetic diseases, and develop targeted therapies. For instance, a scientist investigating a bacterial outbreak can use mass spectrometry to determine the specific strain responsible, allowing for rapid and effective treatment.
Archaeologists rely on mass spectrometry to uncover clues about ancient civilizations. By measuring isotope ratios in pottery, bones, or artifacts, they can trace trading routes, determine diets, and even establish family connections. For example, analyzing the strontium isotope ratios in ancient Egyptian mummies has provided evidence of immigration and cultural exchange.
Environmental scientists harness mass spectrometry to monitor pollutants, track the flow of nutrients, and understand the impact of climate change. By measuring the isotope ratios of water, soil, or air samples, they can identify the sources and pathways of contamination and develop strategies to mitigate environmental degradation.
In summary, mass spectrometry is an indispensable tool that allows scientists to separate, identify, and weigh isotopes, unlocking valuable information about the world around us. From deciphering medical mysteries to unraveling historical enigmas and safeguarding our planet, this technique continues to revolutionize our understanding of the micro and macrocosm.
Nuclear Magnetic Resonance (NMR) Spectroscopy: Unraveling the Chemical Environment of Atoms
In the realm of chemistry, the spatial arrangement of atoms within molecules holds the key to deciphering their intricate properties. Enter Nuclear Magnetic Resonance (NMR) spectroscopy, a powerful analytical technique that empowers chemists to peer into the chemical environment of atoms, revealing the secrets of molecular structure.
NMR spectroscopy harnesses the magnetic properties of atomic nuclei, such as hydrogen, carbon, and nitrogen, which possess a characteristic property known as nuclear spin. When these nuclei are placed in a strong magnetic field, they align themselves either parallel or antiparallel to the field. By applying radiofrequency pulses, these nuclei are perturbed, causing them to absorb energy and flip their alignment. The energy absorbed is characteristic of the specific nucleus and its chemical environment.
By analyzing the pattern of radiofrequency absorption, scientists can identify and determine the chemical environment of each type of atom within a molecule. This information provides invaluable insights into the molecular structure, including the connectivity of atoms and the presence of functional groups. NMR spectroscopy is particularly adept at distinguishing between different types of protons within complex molecules, making it a versatile tool for characterizing organic compounds.
For instance, in the study of proteins, NMR spectroscopy has played a crucial role in determining the three-dimensional structure of these complex biomolecules. The technique has also been instrumental in unraveling the structure and dynamics of DNA, the blueprint of life. Additionally, NMR spectroscopy has found applications in medicine, where it aids in diagnosing diseases and monitoring treatment responses.
In conclusion, NMR spectroscopy offers a non-invasive window into the molecular world, allowing chemists to probe the chemical environment of atoms and unveil the intricacies of molecular structure. Its ability to elucidate complex molecular systems has made it an indispensable tool in a wide range of scientific disciplines, from chemistry and biology to medicine and materials science.
Radioactive Decay Rates: A Constant Process
Radioactive isotopes are atoms with an unstable nucleus, causing them to undergo a process called radioactive decay. During this process, the nucleus emits particles or energy, transforming into a more stable isotope. Each radioactive isotope has a specific decay rate, which determines how quickly it decays.
The decay rate is constant and unaffected by external factors such as temperature, pressure, or chemical reactions. The half-life of an isotope is the time it takes for half of its radioactive nuclei to decay. Every isotope has a unique half-life, which provides valuable information about its stability and decay behavior.
The decay constant is another measure of radioactive decay rate. It represents the fraction of radioactive nuclei that decay per unit time. The decay constant is related to the half-life by the following equation:
decay constant = (ln 2) / half-life
Exponential Decay:
Radioactive nuclei decay in an exponential manner, meaning the rate of decay decreases exponentially over time. This gradual decay pattern is illustrated by the exponential decay curve, which shows the fraction of radioactive nuclei remaining as a function of time.
- Initially: A radioactive sample contains a large number of radioactive nuclei, resulting in a high decay rate.
- Over time: As nuclei decay, the number of radioactive nuclei decreases, leading to a lower decay rate.
- Asymptotically: The fraction of radioactive nuclei approaches zero as time progresses, indicating that the sample approaches stability.
Optical Spectroscopy: Unlocking the Secrets of Energy Levels
In the realm of atomic and molecular science, optical spectroscopy emerges as a powerful tool, unveiling the hidden mysteries of energy levels that shape the very foundation of matter. Through the intricate dance of light and matter, this technique offers profound insights into the inner workings of atoms and molecules, revealing their secrets and unlocking a treasure trove of knowledge.
Imagine a vibrant symphony of light, illuminating the molecular landscape. As photons of light interact with atoms and molecules, they can either be absorbed or emitted, triggering a cascade of energy transitions that hold the key to understanding their internal structures.
Optical spectroscopy harnesses this interplay of light and matter to measure the specific energy levels of these tiny building blocks. By analyzing the wavelengths of light absorbed or emitted, scientists can map out the distinct energy levels that characterize each element or compound.
This technique has revolutionized our understanding of atomic and molecular structures. For instance, by shining light on a sample of hydrogen gas, scientists can observe the characteristic wavelengths emitted as electrons transition between specific energy levels, providing a detailed blueprint of the hydrogen atom’s energy structure.
Moreover, optical spectroscopy extends its analytical prowess to complex molecules. By dissecting the absorption and emission spectra of organic compounds, researchers can identify functional groups, elucidate molecular conformations, and even unravel the intricate mechanisms of biological processes.
In the realm of materials science, optical spectroscopy plays a pivotal role in characterizing the electronic properties of semiconductors and insulators. By measuring the energy gaps between valence and conduction bands, scientists can tailor materials for specific applications, such as solar cells and optoelectronic devices.
Optical spectroscopy’s versatility extends beyond the laboratory. It has found widespread applications in diverse fields, including astrophysics, archaeology, and environmental science. By analyzing the light emitted or absorbed by celestial objects, astronomers glean valuable insights into the composition and evolution of stars, galaxies, and planets.
In archaeology, optical spectroscopy aids in the identification and dating of ancient artifacts. By examining the light absorption properties of pigments and materials, researchers can uncover valuable information about the age, origin, and cultural significance of historical treasures.
In environmental science, optical spectroscopy provides a non-invasive tool for monitoring air and water quality. By detecting the specific wavelengths of light absorbed or emitted by pollutants, scientists can quantify their concentrations and assess their impact on ecosystems.
In essence, optical spectroscopy serves as a multifaceted key, unlocking the enigmatic realms of atomic and molecular energy levels. Through the prism of light, this technique illuminates the hidden architectural blueprints of matter, paving the way for countless scientific breakthroughs and practical applications that shape our world.