Isotopes: Unveiling The Nuances Of Atomic Identity And Chemical Behavior
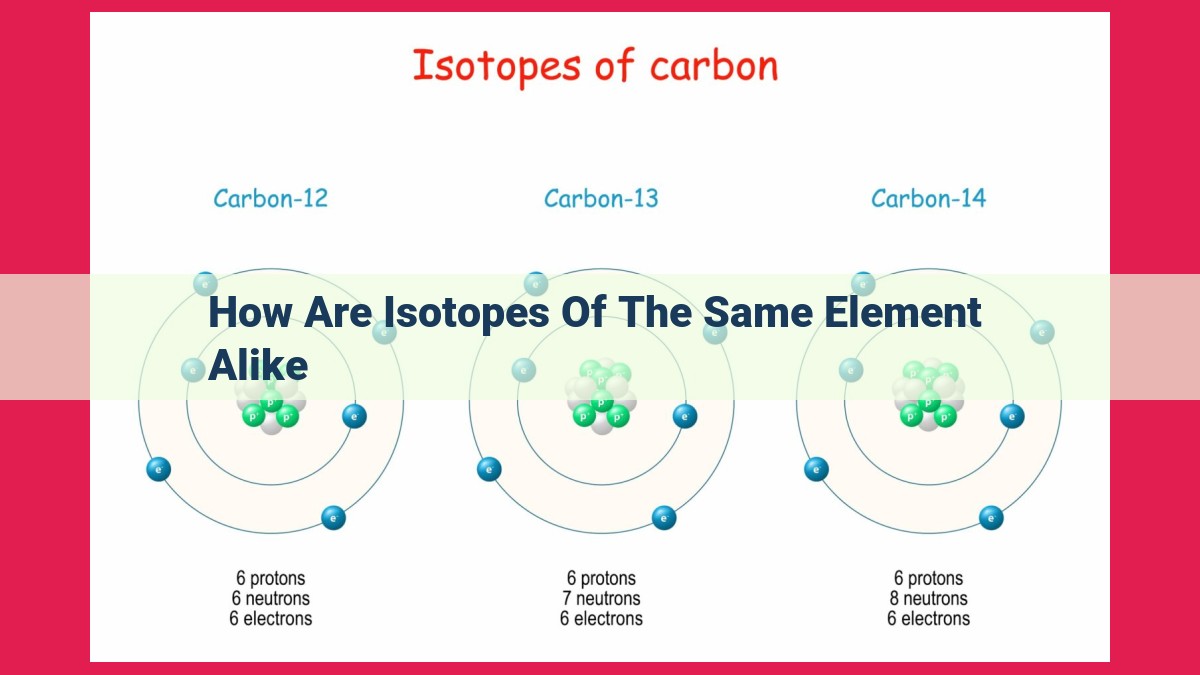
Isotopes of the same element share identical atomic numbers, indicating the same number of protons and electrons, and thus the same chemical properties. They differ in mass number due to varying neutron counts, leading to slight variations in physical properties. However, their electronegativity, valence electrons, and reactivity remain constant, enabling them to participate in similar chemical reactions.
Atomic Number: The Defining Characteristic of an Element
In the vast tapestry of elements that weave together the fabric of our universe, each possesses a unique identity defined by its atomic number. This enigmatic number, imprinted within the heart of every atom, reveals the fundamental essence of an element and governs its destiny.
The atomic number represents the number of protons, the tiny, positively charged particles that reside in the atom’s nucleus. Like a celestial lighthouse, the atomic number illuminates the element’s position on the periodic table, dictating its chemical properties and setting it apart from all others.
Furthermore, the atomic number exerts a profound influence on the number of electrons, the negatively charged particles that dance around the nucleus. The harmony between protons and electrons is essential for the atom’s stability and reactivity. With equal numbers of protons and electrons, the atom exists in a state of electrical neutrality, balancing the forces that bind it together.
Mass Number: Unveiling the Distinction of Isotopes
Every atom consists of a nucleus, the heart of the atom, which houses protons and neutrons. The number of protons, known as the atomic number, defines the element’s identity. Alongside protons, the nucleus also contains neutrons, neutral particles that add to the atom’s mass. The mass number is the sum of both protons and neutrons in an atom.
Isotopes, intriguing variations of the same element, emerge when atoms share an identical atomic number but differ in their neutron count. This difference in neutron number alters the mass number of each isotope. For instance, carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. All three isotopes possess six protons, but they vary in neutron number, resulting in mass numbers of 12, 13, and 14, respectively.
The distinction in mass numbers among isotopes arises from the varying neutron content. Neutrons, despite their lack of electrical charge, contribute significantly to an atom’s mass. By altering the number of neutrons while maintaining the same number of protons, isotopes of the same element exhibit differing mass numbers. This variation in mass number provides the foundation for distinguishing between isotopes and understanding their unique properties.
Properties: Similarities and Nuances
Like family members who share common traits yet possess unique qualities, isotopes of the same element exhibit similarities and differences in their properties.
Physical Properties:
Isotopes share similar physical properties like melting point, boiling point, and density. These properties are primarily influenced by the atomic number and the electron configuration, which remain constant within isotopes of the same element. The mass number contributes minimally to these properties.
Chemical Properties:
Chemically, isotopes behave similarly due to their identical number of protons and electrons. Their electronegativity and valence electrons remain unchanged, influencing their reactivity with other elements. However, slight variations in some chemical properties may occur due to differing neutron numbers.
Mass Effects:
While most properties are largely unaffected by neutron number, mass-dependent properties, such as diffusion rate and isotope fractionation, can show variations. Isotopes with higher mass numbers tend to diffuse slower and experience preferential fractionation in certain processes.
Reactivity and Stability: Chemical Behavior of Isotopes
Reactivity: The Dance of Electrons
Isotopes of the same element share the same number of electrons, determining their electronegativity and valence electrons. These factors heavily influence an element’s reactivity. Electronegativity measures an atom’s ability to attract electrons, while valence electrons are those in the outermost shell, which participate in chemical bonding. Since isotopes have identical electron configurations, their reactivity remains remarkably similar.
Stability: The Delicate Neutron-Proton Balance
The stability of isotopes depends on the neutron-to-proton ratio. This ratio plays a crucial role in the nucleus’s stability. A higher neutron-to-proton ratio leads to greater stability, as neutrons act as a buffer between the positively charged protons, reducing the repulsive electrostatic force. Isotopes with an unfavorable neutron-to-proton ratio may undergo radioactive decay to reach a more stable configuration.
Diverse Applications of Isotopes: Unlocking the Power for Medical, Scientific, and Industrial Advancements
Beyond the realm of fundamental chemistry, isotopes hold immense practical significance in various fields. Their distinct properties and characteristics have led to a multitude of applications that have revolutionized our understanding of the world and empowered us to address critical challenges.
Medical Marvels: Diagnosis and Treatment Enhanced
In the medical arena, isotopes have become indispensable tools for diagnosing and treating a wide range of diseases. Radioactive isotopes, with their controlled emission of particles and energy, enable imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). These non-invasive procedures provide detailed images of the body’s physiological processes, aiding in the diagnosis of cancer, heart disease, and other conditions.
Isotopes also serve as potent agents for medical treatments. In radiation therapy, high-energy radiation from isotopes such as cobalt-60 and gamma rays is used to shrink or destroy cancerous cells. Radioisotope therapy involves the administration of radioactive isotopes that specifically target diseased tissues, delivering targeted radiation for maximum effectiveness.
Scientific Exploration: Unraveling the Tapestry of Time and Nature
In the realm of scientific research, isotopes play a fundamental role in unraveling the secrets of the past and present. Radioactive dating techniques, such as carbon-14 dating, allow scientists to determine the age of ancient artifacts, fossils, and geological formations. By measuring the decay of isotopes over time, researchers can construct detailed chronologies of events that span millions of years.
Isotopes also serve as tracers in scientific studies, enabling the tracking of molecules and elements through complex systems. By tagging substances with distinctive isotopes, researchers can monitor their movement, distribution, and interactions within living organisms, ecosystems, and industrial processes. This knowledge aids in understanding fundamental biological processes, environmental dynamics, and industrial efficiency.
Industrial Ingenuity: Enhancing Safety and Efficiency
In the industrial sector, isotopes find practical applications in various fields. Tracer elements are used in mining and oil exploration to track the movement of fluids and gases, aiding in resource extraction and environmental monitoring. Radiation shielding materials, often composed of isotopes such as lead and uranium, protect workers and equipment from harmful radiation in nuclear power plants, medical facilities, and industrial settings.
The versatility of isotopes continues to fuel innovation and progress across multiple disciplines. Their unique properties and the ability to track and manipulate them have opened up new avenues for scientific exploration, medical advancements, and industrial efficiency. As our understanding of isotopes deepens, we can expect even more transformative applications that will shape the future of medicine, science, and industry.