Iron’s Proton Count: Unraveling The Heart Of An Essential Metal
How Many Protons Does Iron Have?
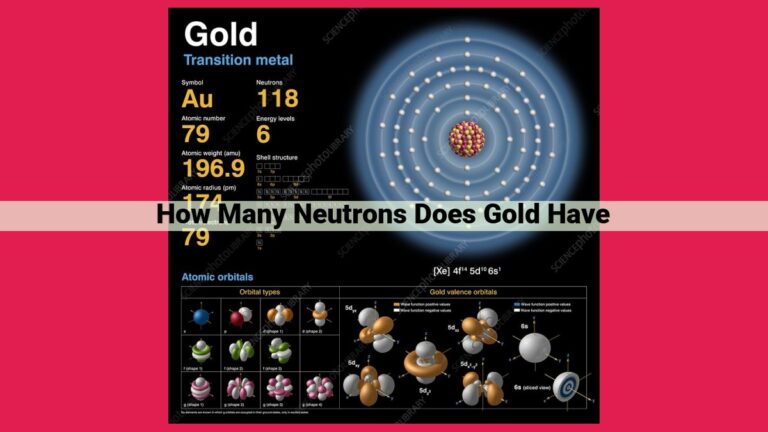
Iron, an essential transition metal, boasts an atomic number of 26, signifying the presence of 26 protons in its nucleus. This number of protons coincides with the number of electrons orbiting the nucleus, contributing to iron’s distinct properties. Iron’s atomic structure consists of protons and neutrons nestled within the nucleus, while electrons occupy designated shells surrounding it. As a result, iron exhibits strength, ductility, and valuable electrical and thermal conductivity, making it indispensable in various industries.
Iron: An Element of Strength and Versatility
In the realm of elements, iron stands tall as a transition metal renowned for its strength, ductility, and versatility. Its unique atomic structure and properties make it indispensable in a myriad of applications.
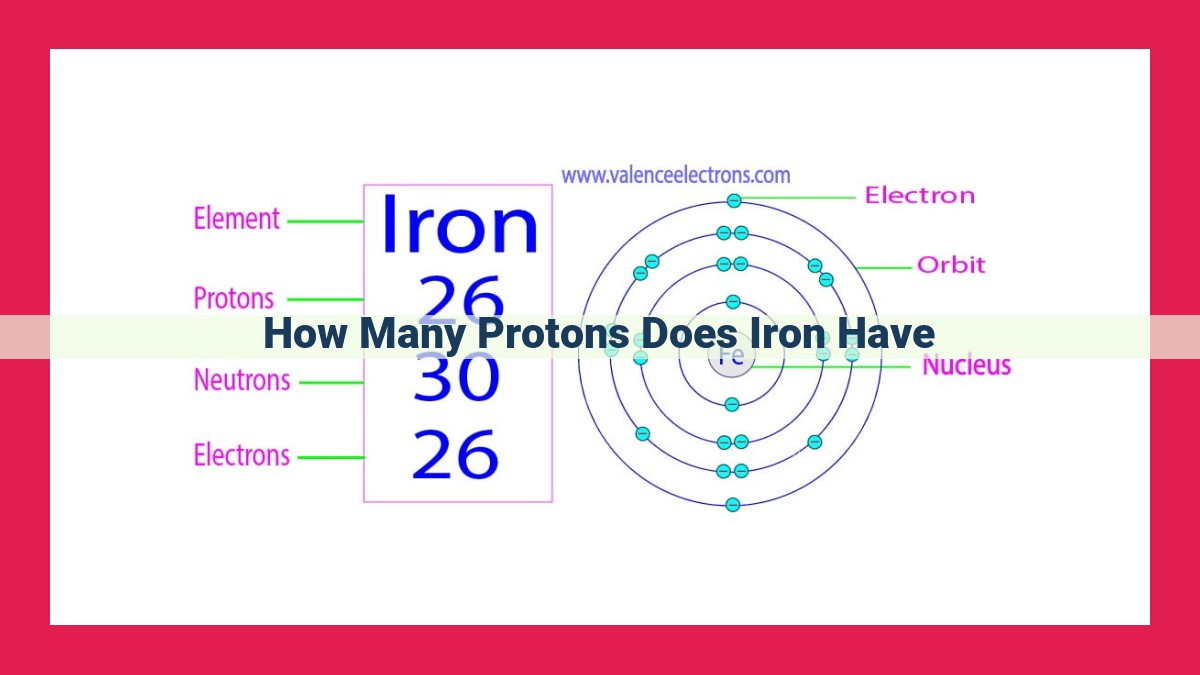
At the heart of every iron atom lies its nucleus, a bustling hub of protons and neutrons. The atomic number of an element, denoted by the symbol Z, represents the number of protons within its nucleus. In the case of iron, Z equals 26, indicating the presence of 26 protons within its nucleus.
Each proton carries a positive electric charge, balanced by an equal number of negatively charged electrons. These electrons orbit the nucleus in concentric layers known as shells. Iron’s 26 electrons occupy four shells, with the outermost shell containing two electrons.
This atomic arrangement bestows iron with valuable properties. Its strength and ductility make it an ideal material for construction, automotive parts, and machinery. Iron is also an excellent conductor of heat and electricity, finding applications in electrical components, cookware, and thermal systems.
In the realm of human health, iron plays a crucial role in oxygen transport. It is an essential component of hemoglobin, the protein responsible for carrying oxygen throughout the body. Deficiency of iron can lead to anemia, characterized by fatigue and pale skin.
Iron’s versatility extends beyond its physical properties. It is also an important nutrient for plants, playing a key role in photosynthesis and plant growth. In industry, iron is used in the production of steel, alloys, and pigments.
From the depths of its atomic structure to its wide-ranging applications, iron remains a testament to the remarkable diversity of elements that shape our world. Its strength, versatility, and vital role in human health and industry make iron an indispensable element in our lives.
The Protonic Core of Iron: Unraveling the Nucleus’s Secrets
Protons: The Building Blocks of Iron’s Identity
In the realm of atoms, *protons* reign supreme as the defining characteristic of each element. They reside at the very heart of the atom’s nucleus, their presence denoted by the *atomic number* – a unique identifier assigned to every element. For iron, this crucial number is 26, signifying the presence of 26 protons within its nucleus. These protons, the positive counterparts of electrons, determine an atom’s chemical properties and its position on the periodic table.
Iron’s Nuclear Ensemble: Protons and Neutrons
Within the confines of iron’s nucleus, protons aren’t solitary dwellers; they share their domain with their counterparts, *neutrons* – particles devoid of electric charge. The number of protons and neutrons in an atom’s nucleus is often equivalent, making iron’s nucleus home to 26 neutrons as well.
This nuclear ensemble orchestrates the element’s fundamental stability. Protons, with their positive charges, repel each other, but neutrons, acting as neutral mediators, bridge the gap and foster cohesion within the nucleus. This delicate balance ensures iron’s stability, allowing it to exist as a distinct and recognizable element.
Iron’s Intricate Symphony: Delving into Its Atomic Structure
Imagine atoms as a miniature solar system, with protons and neutrons as the massive nucleus and electrons gracefully orbiting around them. Iron, a ubiquitous element from our planet’s core to the stars, boasts a captivating atomic structure.
The Nucleus: A Compact Core
Nestled at the heart of an iron atom lies its nucleus, a dense bundle of positively charged protons and neutral neutrons. The number of protons in an atomic nucleus defines an element’s identity. For iron, this number is 26, signifying its atomic number. Within the nucleus, protons and neutrons cohabitate in a delicate balance, each contributing to the atom’s overall mass.
Electron Orbits: A Dance Around the Nucleus
Surrounding the nucleus, 26 electrons engage in an enchanting waltz. Like planets in their orbits, these electrons occupy specific shells or energy levels. The electrons closest to the nucleus, the inner shell, are the most tightly bound and possess the lowest energy. As we venture further from the nucleus, the shells become larger, and electrons gain more energy.
The electron configuration of iron, with 26 electrons, follows a fascinating pattern:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s²
This indicates that the first two electrons occupy the 1s shell, followed by two in the 2s, six in the 2p, two in the 3s, six in the 3p, six in the 3d, and finally, two in the 4s shell. The 3d subshell, with six electrons, is particularly significant, contributing to iron’s remarkable properties.
Iron: An Essential Element with Remarkable Properties
Properties of Iron:
Iron, an indispensable transition metal, boasts an array of valuable properties that have rendered it a cornerstone of human civilization. Its strength and ductility make it an ideal material for countless applications, from towering skyscrapers and sturdy bridges to intricate surgical instruments.
Iron’s durability allows it to withstand wear and tear, ensuring its suitability for long-lasting structures. Its malleability enables it to be shaped into various forms without breaking, further expanding its uses.
Beyond its structural applications, iron plays a vital role in conducting heat and electricity. This property makes it useful for electrical components, transformers, and cooking utensils. Iron’s ability to transfer heat efficiently makes it an excellent choice for heating systems and cookware.
In addition to these practical uses, iron holds significance in our bodies and the natural world. It is an essential component of hemoglobin, the protein that carries oxygen in red blood cells. Its presence in chlorophyll, the green pigment in plants, enables photosynthesis, the process by which plants convert sunlight into energy.
Overall, iron stands as a remarkable element with properties that have shaped human history and continue to drive technological advancements and sustain life on Earth.