Iron (Fe): An Essential Transition Metal For Life
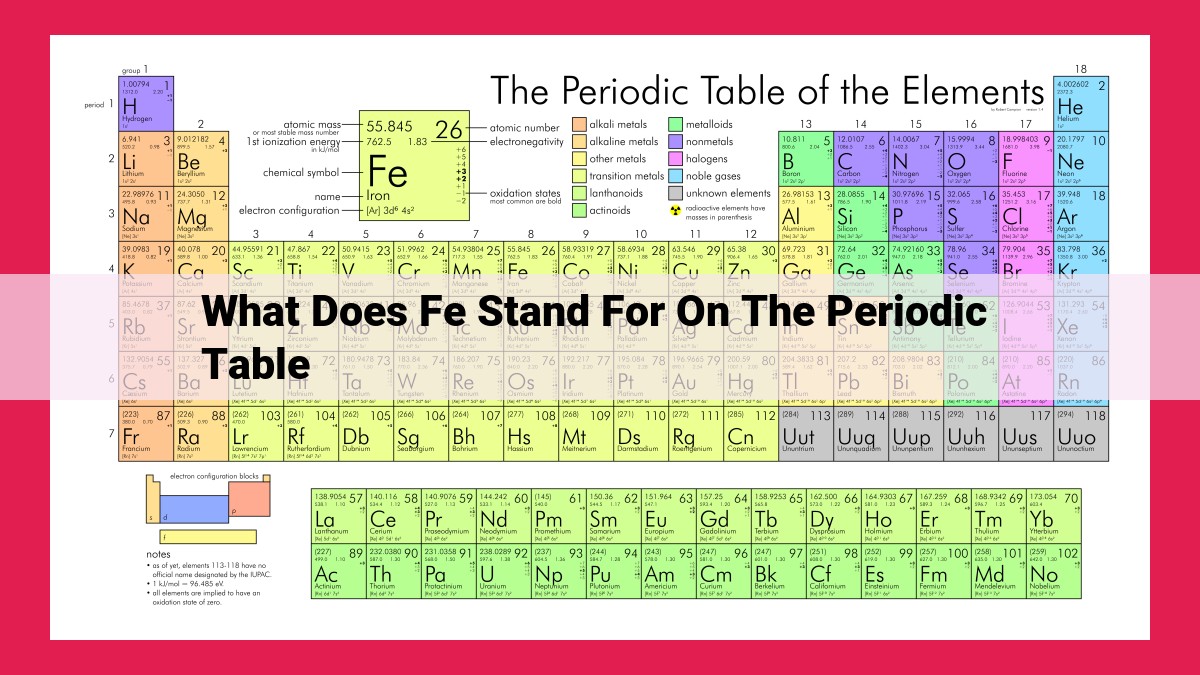
Fe denotes iron on the periodic table, derived from its Latin name “ferrum.” With atomic number 26, iron is a transition metal in the fourth period and Group 8. Its abundance in the Earth’s crust and its vital role in biological systems, particularly in hemoglobin, make it essential for life.
Exploring the Enigma of Iron: Unraveling its Symbol, History, and Significance
The International Symbol: Fe
In the realm of chemistry, each element possesses a unique symbol, a shorthand representation that serves as its identity. For iron, the symbol is Fe, a legacy that traces its roots back to the ancient Latin name for the metal: Ferrum. This abbreviation has endured the test of time, becoming the universally recognized symbol for iron, a testament to its enduring significance.
The Origin of “Fe”: A Latin Legacy
The Latin word Ferrum had a profound impact on the development of the symbol Fe. In the 18th century, Swedish chemist Jöns Jakob Berzelius proposed a system of chemical symbols based on the first letter or two of the Latin names of elements. This system gained widespread acceptance, solidifying the use of Fe as the symbol for iron.
Atomic Number 26
- Define atomic number and its role in identifying elements.
- State the atomic number of iron as 26 and explain its implications.
Atomic Number 26: Delving into the Identity of Iron
Within the vast tapestry of elements that make up our world, iron stands out as a cornerstone of both nature and human existence. Its unique characteristics and pivotal role in countless processes have earned it a place of prominence in the periodic table, where it resides at atomic number 26.
Defining Atomic Number
Atomic number, a fundamental concept in chemistry, refers to the number of protons found within the nucleus of an atom. It serves as a unique identifier for each element, assigning it a specific place on the periodic table. Each element’s atomic number is immutably determined and cannot be altered through chemical reactions.
Iron’s Atomic Number of 26
Iron, with its atomic number of 26, signifies the presence of 26 protons within its nucleus. This defining characteristic places iron in a specific location within the periodic table, among the 3d transition metals and in the d-block of elements.
Implications of Iron’s Atomic Number
Iron’s atomic number has profound implications for its chemical properties and behavior. The number of protons determines the number of electrons that surround the nucleus, which in turn influences an element’s chemical reactivity and bonding capabilities. For iron, its 26 protons result in 26 electrons, distributed across its various energy levels.
Additionally, the atomic number plays a crucial role in understanding iron’s position in the periodic table. Its placement within the fourth period indicates that iron has four energy levels or electron shells. Furthermore, its presence in Group 8 (also known as the iron triad) highlights iron’s similarities to other transition metals such as cobalt and nickel.
Transition Metals: Unveiling the Versatile Nature of Iron
Transition metals occupy a prominent position in the periodic table, captivating scientists and engineers with their multifaceted properties. Iron, with its atomic number of 26, stands as a shining example of this remarkable class of elements.
Located in the d-block of the periodic table, transition metals are characterized by their partially filled d-orbitals. These orbitals house electrons that are not involved in chemical bonding, giving rise to distinctive properties that set them apart from other elements.
Transition metals are typically hard and malleable, making them ideal for a wide range of applications. They are also excellent conductors of heat and electricity, ensuring their widespread use in electronics. One of the most notable features of transition metals is their ability to form complexes, which involve the sharing of electrons between the metal ion and other atoms or molecules. This property is crucial in many biological processes, including oxygen transport and photosynthesis.
The d-block elements exhibit a vibrant array of colors due to their partially filled d-orbitals. This phenomenon, known as selective absorption, allows transition metals to absorb light of specific wavelengths, resulting in the characteristic hues observed in their compounds.
Overall, the unique properties of transition metals stem from their partially filled d-orbitals, making them essential components in countless technological and biological systems. Iron, as a prominent member of this group, plays a pivotal role in everything from skyscrapers to human bodies, underscoring the versatility and importance of transition metals in our world.
Unraveling Iron’s Periodic Table Secrets: Fourth Period
In the enigmatic world of chemistry, the periodic table serves as a roadmap, guiding us through the vastness of elements. Iron (Fe), a ubiquitous metal with a profound impact on our planet and lives, occupies a distinct position within this intricate tapestry.
The concept of periods in the periodic table refers to the rows running horizontally across the grid. Each period represents an increase in the number of electron shells surrounding the atom’s nucleus. Iron resides in the fourth period, indicating that it possesses four electron shells. These shells, like celestial orbits, accommodate electrons in increasing numbers as you move across the period.
Key Points to Remember:
- The periodic table is an organizational system for elements based on their atomic number, electron configuration, and chemical properties.
- Periods indicate the number of electron shells an element has.
- Iron is located in the fourth period, signifying that it has four electron shells.
Group 8
- Introduce the concept of groups in the periodic table.
- Identify iron as part of Group 8, also known as the iron triad.
Group 8: The Iron Triad
In the periodic table, elements are arranged into vertical columns called groups. These groups share similar chemical properties, providing valuable insights into their behavior and characteristics. One such group is Group 8, also known as the iron triad.
The members of Group 8—iron (Fe), cobalt (Co), and nickel (Ni)—are captivating metals that share intriguing attributes. These elements are often found together in nature and exhibit remarkable similarities in their chemical and physical properties.
Iron, in particular, stands out as a d-block element, meaning it has partially filled d-electron orbitals. This unique electronic configuration contributes to iron’s role as a transition metal, renowned for its versatile bonding capabilities, forming diverse compounds with varying oxidation states.
The prevalence of iron in the Earth’s crust is astonishing. It’s the fourth most abundant element, surfacing in various minerals and ores. From the vast iron ore deposits found in countries like Brazil and Australia to the intricate pyrite crystals adorning geological specimens, iron’s presence is undeniable.
Furthermore, iron plays a crucial role in life. Its involvement in biological processes is indispensable, particularly as a component of hemoglobin. This remarkable protein facilitates oxygen transport throughout the body, ensuring the vitality of all living organisms.
The iron triad—iron, cobalt, and nickel—holds immense importance in diverse industrial applications. They are widely employed in the production of steel, alloys, batteries, and countless other products. Their magnetic properties and corrosion resistance make them invaluable in a multitude of technological advancements.
Understanding the properties of Group 8 elements, including iron, provides a solid foundation for exploring the fascinating world of chemistry. By unraveling the intricacies of their electronic structures, abundance, and biological significance, we deepen our appreciation for the elements that shape our world.
d-Block
- Discuss the characteristics of d-block elements, particularly their partially filled d-orbitals.
- Explain the transition metal nature of iron.
A Deeper Dive into Iron: Exploring the d-Block and Its Transition Metal Nature
In the heart of the periodic table lies a realm of elements known as the d-block, where iron (Fe) resides. These elements are characterized by their partially filled d-orbitals, which give rise to their unique transition metal properties.
Transition Metal Magnetism
One defining characteristic of transition metals is their ability to exhibit magnetism. This magnetism originates from the presence of unpaired electrons in the d-orbitals. Iron, with its 26 electrons arranged in the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶ 4s², has four unpaired electrons in its d-orbitals, making it a ferromagnetic material. This means that iron atoms align their magnetic fields in the same direction, creating a strong magnetic force.
Versatile Reactivity
Another hallmark of d-block elements is their diverse reactivity. The partially filled d-orbitals allow for variable oxidation states, enabling iron to form various compounds with different properties. For instance, iron commonly exists in both the Fe(II) and Fe(III) oxidation states, each exhibiting distinct chemical behavior and playing essential roles in biological systems.
Iron in the Body: A Vital Element
The transition metal nature of iron is not just a matter of scientific curiosity; it has profound implications for life on Earth. Iron is an essential nutrient, forming the core of hemoglobin, the oxygen-carrying protein in our blood. Without iron, hemoglobin cannot effectively transport oxygen, leading to anemia and other health issues. Additionally, iron plays a crucial role in energy production, cell growth, and other metabolic processes.
Iron in the Earth’s Crust: A Bulwark of Stability
Iron’s abundance in the Earth’s crust, where it constitutes about 5%, has shaped our planet’s history and development. It is found in a variety of minerals and ores, including magnetite (Fe₃O₄), hematite (Fe₂O₃), and limonite (FeO(OH)·nH₂O). The presence of iron in rocks and minerals has influenced the Earth’s magnetic field and contributed to the formation of the Earth’s core.
In essence, the d-block elements, and iron in particular, embody the fascinating world of transition metals. Their unique electronic configurations and transition metal properties have profound implications for magnetism, reactivity, and the very foundations of life and our planet.
Iron: The Earth’s Abundant Metallic Marvel
Most Abundant Metal in the Earth’s Crust
Peering beneath the Earth’s surface, we discover a treasure trove of minerals, and among them, one metal reigns supreme: iron. This ubiquitous element forms the core of our planet and weaves its way through the Earth’s crust, accounting for an impressive 5% of its mass.
Iron’s presence manifests in a plethora of minerals and ores, each bearing a unique story of geological formation. Hematite, with its lustrous red hue, emerges as the most prevalent iron ore, captivating the imagination with its sedimentary legacy. Magnetite, a magnetic wonder, aligns its atoms in a symphony of order, granting it its distinctive attraction to magnets. And Limonite, an earthy brown mineral, whispers tales of its hydrated origin.
These iron-rich treasures are scattered across the globe, adorning landscapes with their diverse forms. From the vast iron ore deposits of Minnesota and Australia to the ancient magnetite outcrops of Sweden, iron’s presence is both abundant and awe-inspiring.
Iron: The Essential Mineral for Life
Iron: A Vital Player in Biological Processes
Iron, denoted by the chemical symbol Fe, plays a crucial role in our bodies, enabling various biological processes that sustain life. This essential mineral forms the core of hemoglobin, the protein in our red blood cells that transports oxygen throughout our bodies. Without adequate iron, our cells would be starved of this vital gas, leading to fatigue, weakness, and even more severe health issues.
Its Role in Hemoglobin
Hemoglobin is a complex protein that carries oxygen molecules from our lungs to every nook and cranny of our bodies. Each hemoglobin molecule contains four iron atoms bound to a heme group, a flat ring-like structure. When oxygen is present, it binds to the iron atoms, forming oxyhemoglobin. This oxygenated hemoglobin then travels through our bloodstream, delivering oxygen to our cells.
Beyond Hemoglobin
In addition to its critical role in oxygen transport, iron is also involved in other vital processes. It facilitates energy production through its role in the electron transport chain in mitochondria, the energy powerhouses of our cells. Iron also plays a part in cell growth, DNA synthesis, and immune function.
Deficiency and Consequences
Iron deficiency can have devastating consequences, leading to anemia, a condition characterized by a reduced number of healthy red blood cells. Symptoms of anemia include fatigue, shortness of breath, and pale skin. In severe cases, iron deficiency can even impair cognitive function and lead to heart problems.
Iron is an essential mineral for human life, playing a vital role in oxygen transport, energy production, and cell growth. Its presence in hemoglobin enables oxygen to reach every cell in our bodies, keeping us healthy and energetic. Understanding the importance of iron and maintaining adequate levels of this mineral is crucial for our overall well-being.