Essential Guide To Ionization Enthalpy: Unraveling The Key To Atomic And Chemical Properties
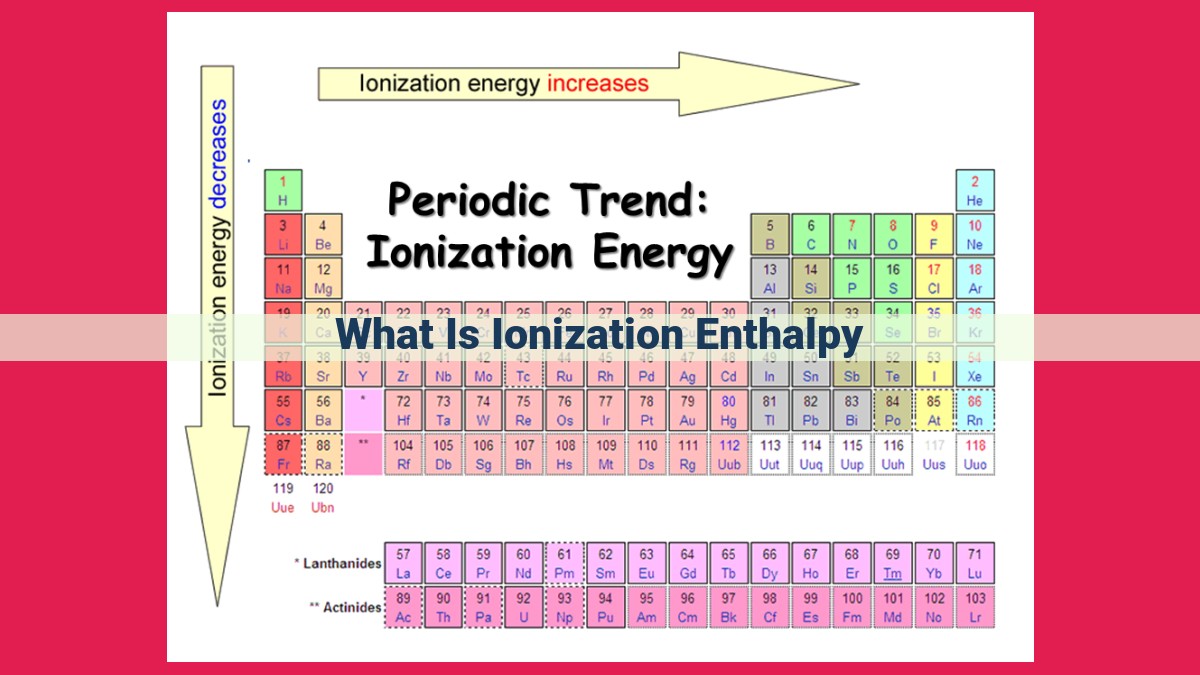
Ionization enthalpy is the energy needed to remove an electron from an isolated gaseous atom. First ionization enthalpy refers to the energy required to remove the first electron, and it is directly related to the atom’s size, nuclear charge, screening effect, and electronic configuration. Successive ionization enthalpies increase as more electrons are removed, reflecting the increased effective nuclear charge. Ionization enthalpy plays a crucial role in determining the element’s reactivity, electronegativity, ionic compound formation, and chemical bonding characteristics.
Understanding Ionization Enthalpy: The Key to Unraveling Chemical Properties
Embark on a fascinating journey into the realm of ionization enthalpy, a fundamental concept that unlocks the secrets of chemical elements. Ionization enthalpy, the energy required to remove an electron from an isolated gaseous atom, holds the key to understanding the reactivity, electronegativity, and bonding behavior of elements.
Definition:
Simply put, ionization enthalpy is the energy needed to pry an electron from the clutches of an isolated gaseous atom. This process, akin to unzipping a tiny atomic suit, reveals the atom’s inner workings and paves the way for understanding its chemical properties.
Classification of Ionization Enthalpy:
-
First Ionization Enthalpy: This refers to the energy required to remove the first electron from an atom. It provides insights into the atom’s stability and its tendency to lose electrons.
-
Successive Ionization Enthalpies: These represent the energies needed to remove subsequent electrons from an atom. As we remove more electrons, the effective nuclear charge increases, making it progressively more difficult to remove electrons.
Factors Influencing Ionization Enthalpy:
-
Atomic Size: Smaller atoms generally have higher ionization enthalpies as the electrons are held more tightly by the nucleus.
-
Nuclear Charge: Elements with a greater number of protons (positive charges) have higher ionization enthalpies.
-
Screening Effect: Inner electrons shield the outer electrons from the nucleus, reducing the effective nuclear charge and thus lowering ionization enthalpy.
-
Electronic Configuration: Half-filled or fully filled electron orbitals have higher ionization enthalpies due to exchange energy and Hund’s rule.
Applications of Ionization Enthalpy:
-
Determining Element Reactivity and Electronegativity: Ionization enthalpy indicates an element’s reactivity and ability to attract electrons.
-
Predicting Ion and Ionic Compound Formation: The successive ionization enthalpies help predict the stability of ions and the formation of ionic compounds.
-
Understanding Chemical Bonding and Molecular Structures: Ionization enthalpy provides insights into chemical bonding and the stability of molecular structures.
Understanding Ionization Enthalpy
When you hear the term “ionization enthalpy,” it may sound like a complex scientific concept. But in reality, it’s quite straightforward and plays a crucial role in understanding the behavior of atoms. In this article, we’ll unravel the mystery of ionization enthalpy and explore its fascinating applications.
First Ionization Enthalpy: The Gateway to Understanding
Ionization enthalpy is the energy required to remove an electron from an isolated gaseous atom. Think of it as the effort needed to “kick” an electron out of an atom. The first ionization enthalpy is the energy required to remove the first electron. It’s a fundamental property of an atom that tells us how strongly it holds onto its electrons.
The first ionization enthalpy is directly related to the ionization enthalpy of an atom. Ionization enthalpy is the energy required to remove an electron from any orbital of an atom. The first ionization enthalpy is a specific case where the electron is removed from the outermost orbital.
By measuring the first ionization enthalpy of elements, we can gain valuable insights into their reactivity. It helps us predict whether an element is likely to donate or accept electrons in chemical reactions. For instance, elements with low first ionization enthalpies tend to lose electrons easily, making them good reducing agents. Conversely, elements with high first ionization enthalpies hold their electrons tightly and act as good oxidizing agents.
Factors Influencing First Ionization Enthalpy
Several factors influence the first ionization enthalpy of an element:
-
Atomic Size: Smaller atoms have higher first ionization enthalpies because the electrons are closer to the nucleus and more tightly bound.
-
Nuclear Charge: The more protons in an atom’s nucleus, the stronger the attraction for electrons and the higher the first ionization enthalpy.
-
Screening Effect: Inner electrons can shield the nucleus from the outer electrons, reducing their attraction and lowering the first ionization enthalpy.
-
Electronic Configuration: Half-filled or fully filled electron orbitals have higher stability, resulting in higher first ionization enthalpies.
Understanding these factors helps us comprehend the periodic trends in first ionization enthalpies and predict the behavior of elements in different chemical reactions.
In conclusion, understanding the first ionization enthalpy is essential for deciphering the chemical properties of elements and their tendency to form chemical bonds. It provides a foundation for comprehending the reactivity, electronegativity, and bonding behavior of atoms.
Exploring Ionization Enthalpy: Unlocking the Secrets of Successive Ionization
In the realm of chemistry, ionization enthalpy holds a prominent position as a crucial concept that governs the behavior of atoms and ions. When we delve deeper into this fascinating subject, we encounter the concept of successive ionization enthalpies, which sheds light on a fundamental phenomenon that occurs as we remove multiple electrons from an atom.
Successive Ionization Enthalpy: A Step-by-Step Journey
As the name suggests, successive ionization enthalpy refers to the increasing energy required to remove each subsequent electron from an atom. This phenomenon stems from the fact that as we remove more electrons, the remaining electrons become more tightly bound to the nucleus due to the increased effective nuclear charge.
The effective nuclear charge represents the net positive charge experienced by the remaining electrons in an atom. As electrons are removed, the number of protons in the nucleus remains the same, but the number of electrons screening the nucleus decreases. This results in a stronger electrostatic attraction between the nucleus and the remaining electrons, making it more challenging to remove them.
Understanding Effective Nuclear Charge
The concept of effective nuclear charge is vital in comprehending successive ionization enthalpies. It arises from the shielding effect of inner electrons, which reduce the attraction between the nucleus and the outermost electrons. As we remove more electrons from the outer shells, the shielding effect diminishes, leading to an increased effective nuclear charge.
Significance of Successive Ionization Enthalpies
Successive ionization enthalpies provide valuable insights into the electronic structure and chemical reactivity of elements. They assist in predicting the formation of ions and ionic compounds. By examining the trend of successive ionization enthalpies, we can gain insights into the stability and reactivity of various ions.
In conclusion, successive ionization enthalpies offer a deeper understanding of the intricate behavior of atoms as they lose electrons. They reveal the interplay between nuclear charge, electron shielding, and the energy required to remove electrons. By exploring this concept, we unravel the secrets of atomic structure and chemical reactivity, paving the way for further discoveries in the fascinating world of chemistry.
Factors Influencing Ionization Enthalpy
Understanding the Impact on Electron Removal
Ionization enthalpy, the energy required to remove an electron from an atom, is influenced by several key factors. Understanding these factors is essential for comprehending the behavior of atoms and their ability to form ions.
Atomic Size
The size of an atom plays a crucial role in determining its ionization enthalpy. Smaller atoms have higher ionization enthalpies because the electrons are held closer to the nucleus by the stronger electrostatic attraction. As the atomic radius increases, the electrons become more distant from the nucleus, reducing the electrostatic attraction and thus lowering the ionization enthalpy.
Nuclear Charge
The nuclear charge, or the number of protons in the nucleus, directly affects the ionization enthalpy. The more protons, the stronger the electrostatic attraction between the nucleus and the electrons. This increased attraction makes it more difficult to remove an electron, resulting in a higher ionization enthalpy.
Screening Effect
The screening effect refers to the ability of inner electrons to shield outer electrons from the electrostatic attraction of the nucleus. Inner electrons create an electron cloud around the nucleus, which reduces the effective nuclear charge experienced by the outer electrons. This shielding effect lowers the ionization enthalpy of outer electrons.
Electronic Configuration
The electronic configuration of an atom also influences its ionization enthalpy. Atoms with half-filled or fully filled electron orbitals have higher ionization enthalpies. These configurations are more stable and require more energy to remove an electron. Conversely, atoms with unpaired electrons or incomplete orbitals have lower ionization enthalpies due to their instability.
Applications of Ionization Enthalpy: Unlocking the Secrets of Chemistry
Ionization enthalpy, the energy required to remove an electron from an atom, plays a crucial role in determining the reactivity and electronic behavior of elements. Its applications extend far and wide, providing valuable insights into the world of chemistry.
Predicting Reactivity and Electronegativity
The ionization enthalpy of an element is directly related to its reactivity. Elements with lower ionization enthalpies tend to be more reactive, as they lose electrons more easily. This property is essential in understanding the formation of chemical bonds and the reactivity of metals.
Electronegativity, a measure of an atom’s ability to attract electrons, is also influenced by ionization enthalpy. Elements with high ionization enthalpies have a strong hold on their electrons, making them less electronegative. This concept helps explain the formation of ionic and covalent compounds.
Predicting Ion and Ionic Compound Formation
Ionization enthalpy is a key factor in predicting the formation of ions and ionic compounds. When an atom loses or gains electrons, it becomes an ion. The ability of an atom to form a stable ion is directly related to its ionization enthalpy.
Elements with low ionization enthalpies can easily lose electrons, forming cations (positively charged ions). Conversely, elements with high ionization enthalpies have difficulty losing electrons, favoring the formation of anions (negatively charged ions).
Understanding Chemical Bonding and Molecular Structures
Ionization enthalpy provides valuable insights into the formation of chemical bonds and the structures of molecules. The ionization enthalpies of the participating atoms influence the type of bond that is formed (ionic, covalent, or metallic) and the geometry of the molecule.
For example, elements with high ionization enthalpies tend to form covalent bonds by sharing electrons, while those with low ionization enthalpies favor ionic bonds involving the transfer of electrons. Understanding ionization enthalpies helps chemists predict the properties and structures of compounds.