Ionic Vs. Covalent Bonds: Electronegativity Difference And Bond Character
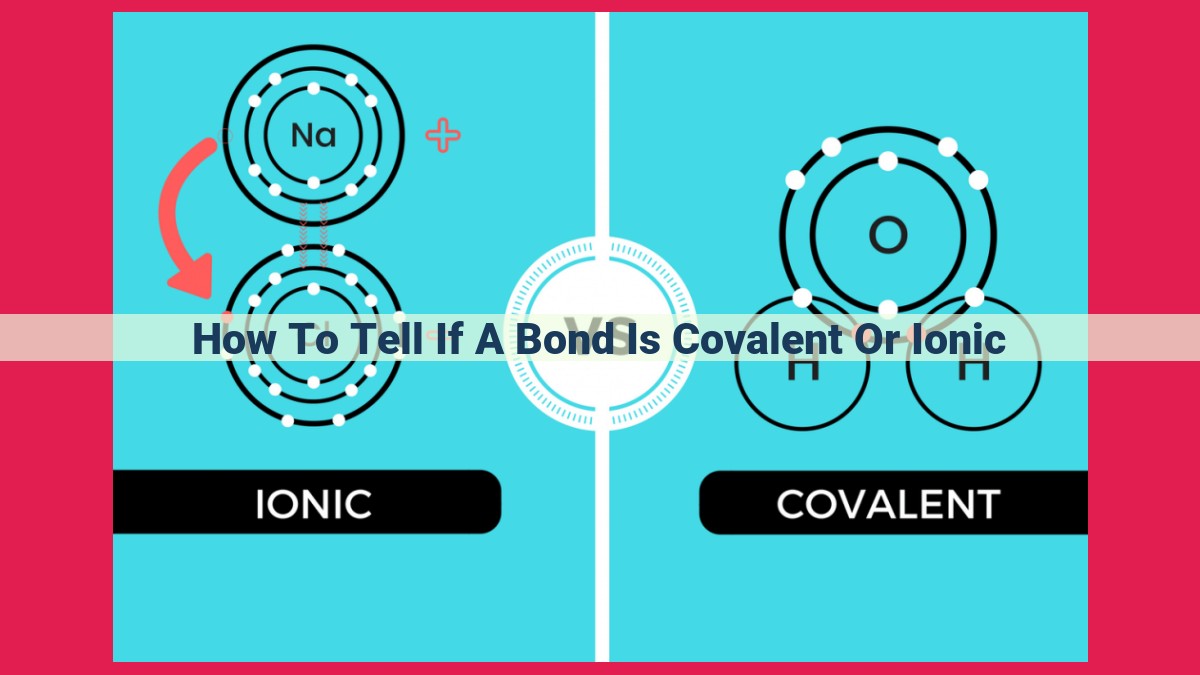
To determine if a bond is covalent or ionic, consider the electronegativity difference between the bonded atoms. If the difference is small (<2.0 on the Pauling scale), the bond is covalent (electrons are shared). If the difference is large (≥2.0), the bond is ionic (electrons are transferred). For covalent bonds, higher electronegativity differences result in more polar bonds. Resonance and hybridization can also affect bond character.
Electronegativity: The Key to Unraveling the Secrets of Chemical Bonds
In the realm of chemistry, understanding the nature of chemical bonds is paramount to comprehending the behavior and properties of matter. Electronegativity, a fundamental concept in chemistry, plays a pivotal role in determining the character and strength of these bonds.
Defining Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons. It reflects the strength with which an atom pulls electrons towards itself in a chemical bond. The Pauling scale is a widely used scale to quantify electronegativity, with values ranging from 0.7 (for cesium) to 4.0 (for fluorine).
Electronegativity and Bond Type
The electronegativity difference between two bonded atoms significantly influences the type of bond formed. When the electronegativity difference is small, the bond tends to be covalent, characterized by the sharing of electrons between the atoms. In contrast, when the electronegativity difference is large, the electrons are more strongly attracted to one of the atoms, resulting in an ionic bond, where complete electron transfer occurs.
Example:
Water (H2O) is a covalent molecule because the electronegativity difference between hydrogen and oxygen is small (1.2). Sodium chloride (NaCl), on the other hand, is an ionic compound due to the large electronegativity difference between sodium and chlorine (2.1).
Electronegativity Difference and Bond Character: Unlocking the Nature of Chemical Bonds
Electronegativity is the key to understanding the character of chemical bonds. It reflects an atom’s ability to attract electrons towards itself. The electronegativity difference between two atoms determines the type of bond formed between them.
Ionic Bonds
When the electronegativity difference between two atoms is high, greater than or equal to 1.7, an ionic bond is formed. In this scenario, the more electronegative atom strips away an electron from the less electronegative atom, resulting in the formation of ions. These ions are held together by strong electrostatic forces. A classic example is sodium chloride (NaCl), where sodium readily donates its electron to chlorine, forming charged ions: Na⁺ and Cl⁻.
Covalent Bonds
On the other hand, when the electronegativity difference is low, less than 1.7, a covalent bond is formed. Here, the atoms share electrons rather than transferring them. The shared electrons create a region of high electron density between the atoms, forming a covalent bond. A prime example is methane (CH₄), where the carbon atom shares its electrons with four hydrogen atoms, forming four covalent bonds.
Examples of Electronegativity Differences and Bond Character
- Hydrogen fluoride (HF): The electronegativity difference is 1.9, indicating an ionic bond with significant charge separation.
- Water (H₂O): The electronegativity difference is 1.3, leading to a polar covalent bond with a partial positive charge on hydrogen and a partial negative charge on oxygen.
- Carbon dioxide (CO₂): The electronegativity difference is 0.8, resulting in a nonpolar covalent bond with no significant charge separation.
Electron Transfer vs. Sharing
Ionic bonds are characterized by the complete transfer of electrons from one atom to another, creating charged ions. The strength of the bond arises from the electrostatic attraction between the oppositely charged ions.
Covalent bonds involve the sharing of electrons between atoms. The strength of the bond stems from the attraction between the positively charged nuclei and the shared electron pairs.
Resonance and Hybridization: Unveiling the Complexities of Chemical Bonds
Beyond the fundamental understanding of electronegativity and its impact on bond character, we delve into the fascinating world of resonance and hybridization to unravel the intricacies of chemical bonds.
Resonance: A Tale of Multiple Structures
Resonance is a concept that introduces us to the idea of multiple Lewis structures for certain molecules. These structures represent the delocalization of electrons across multiple atoms, resulting in a hybrid molecule with characteristics distinct from any single Lewis structure. Resonance impacts both electronegativity and bond polarity.
Hybridization: Modifying Atomic Orbitals
Hybridization involves the mixing of atomic orbitals to create new hybrid orbitals with different shapes and energy levels. This process significantly affects the formation of covalent bonds. By altering the shapes of atomic orbitals, hybridization influences the overlap and strength of the resulting bonds.
The Interplay Between Resonance and Hybridization
The relationship between resonance and hybridization is intricately linked. Resonance can give rise to delocalized electrons, which can participate in hybridization, resulting in the formation of hybrid orbitals with extended delocalization. In turn, this delocalization of electrons through resonance and hybridization affects the molecular geometry and bond polarity of the molecule.
Understanding resonance and hybridization empowers us to decipher the intricate nature of chemical bonds. These concepts provide essential insights into the electronic structure and behavior of molecules, enabling us to unravel the fundamental principles that govern chemical bonding and the properties that arise from these interactions.
Determining Bond Polarity: Unraveling the Electric Dance of Molecules
In the symphony of chemical bonds, electronegativity plays a pivotal role in determining the polarity of the dance between atoms. Bond polarity signifies the uneven distribution of electrons within a covalent bond, a key factor in shaping the molecule’s properties and reactivity.
Electronegativity, measured on the Pauling scale, reflects an atom’s tendency to attract electrons. When atoms with different electronegativities form a bond, the more electronegative atom pulls the shared electron pair closer to itself, creating a partial negative charge. Conversely, the less electronegative atom acquires a partial positive charge. This unequal electron distribution results in a polar covalent bond.
The extent of bond polarity is directly influenced by the electronegativity difference between the bonded atoms. Large electronegativity differences lead to highly polar bonds, approaching the extreme of ionic bonds, where one atom completely transfers an electron to the other. Small electronegativity differences result in nonpolar covalent bonds, where the electrons are shared equally.
The polarity of a bond also influences its ionic character. Ionic character measures the extent to which a bond resembles an ionic bond, with complete electron transfer. Highly polar covalent bonds exhibit significant ionic character, while nonpolar covalent bonds have negligible ionic character.
Understanding bond polarity is crucial for unraveling the intricate tapestry of chemical properties. Polar bonds allow for the formation of intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, which influence molecular structure, solubility, and reactivity. Bond polarity also governs the behavior of molecules in electric fields and plays a vital role in biological processes like protein folding and enzyme catalysis.
In essence, bond polarity is a fundamental concept that illuminates the nature of chemical bonds and their profound impact on molecular behavior. Grasping the interplay between electronegativity, ionic character, and bond polarity empowers us to comprehend the diverse world of chemical substances and their myriad applications.