Ionic Compound Formulas: Balancing Charges For Neutral Compounds
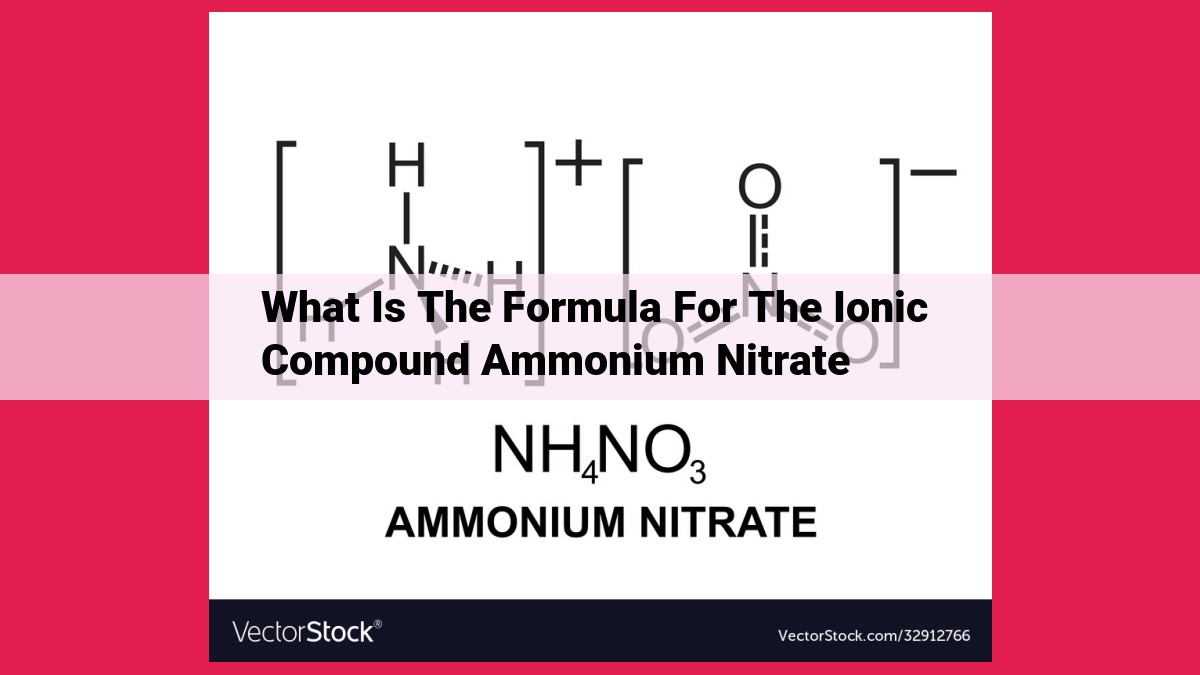
Ionic compounds, composed of positively charged cations and negatively charged anions, form by balancing their charges to create a neutral compound. The formula for ammonium nitrate, NH₄NO₃, is derived from the ammonium ion (NH₄⁺) and the nitrate ion (NO₃⁻). The ammonium ion has a +1 charge, while the nitrate ion has a -1 charge. To balance these charges, one ammonium ion combines with one nitrate ion, resulting in the neutral compound ammonium nitrate. Understanding ionic compound formulas is crucial for comprehending their structure, properties, and reactivity in chemical reactions.
Understanding Ionic Compound Formulas: A Guide to Ions and the Building Blocks of Matter
Every object in our world is made up of tiny building blocks called atoms. Atoms, in turn, are composed of even smaller particles known as ions. When atoms lose or gain electrons, they become charged and are called ions. Ionic compounds are formed when positively charged ions, called cations, combine with negatively charged ions, called anions, to create a neutral compound.
Understanding ionic compound formulas is crucial for several reasons. It helps us:
- Identify and classify different types of ionic compounds
- Predict their properties, such as solubility and melting point
- Describe the chemical reactions they can undergo
- Apply this knowledge in various fields, including medicine, chemistry, and material science
Cations and Anions
- Explanation of cation and anion formation
- Description of positive and negative charges in ions
Cations and Anions: The Dynamic Duo of Ionic Compounds
In the realm of chemistry, ionic compounds dance to the rhythm of positive and negative charges. These compounds, formed by the union of metal and nonmetal elements, rely on the unique characteristics of their atomic structures. When atoms shed or gain electrons, they transform into ions, the building blocks of ionic compounds.
Cations, like knights in shining armor, carry a positive charge due to the loss of one or more electrons. Metals, drawn by their noble aspirations, exhibit a tendency to lose electrons, becoming positively charged cations. Sodium (Na), for instance, readily gives up an electron, transforming into Na⁺, a cation with a single positive charge.
On the opposite end of the spectrum, anions emerge as the queens of the chemical kingdom. These nonmetal atoms have embraced their extra electrons, gaining a negative charge. Chlorine (Cl), with its seven lonely electrons, eagerly accepts an electron, becoming Cl⁻, an anion with a single negative charge.
The dance between cations and anions is orchestrated by their electrostatic attraction. Positive cations are drawn to negative anions, forming ionic bonds that hold them together in a crystal lattice. This arrangement creates stable, electrically neutral compounds, the backbone of many everyday substances.
In the symphony of ionic compounds, cations and anions play a harmonious duet, contributing their positive and negative charges to create a balanced and stable chemical landscape.
**Unveiling the Ammonium Ion: A Treasure Trove of Nitrogen Compounds**
In the realm of chemistry, ionic compounds play a pivotal role in shaping the world around us. Among these ionic wonders, the ammonium ion (NH₄⁺) stands out as a fascinating and versatile entity.
Structure and Properties of the Ammonium Ion
The ammonium ion boasts a fascinating structure, resembling a tiny tetrahedron. At its heart lies a nitrogen atom surrounded by four hydrogen atoms. This unique arrangement gives the ion a positive charge, rendering it a cation. The ammonium ion is a stable and water-soluble species, making it a common sight in aqueous solutions.
Common Nitrogen Compounds Containing Ammonium Ions
The ammonium ion is a key component in a wide array of nitrogen compounds, each with its unique properties and applications. Some of the most notable include:
- Ammonium Chloride (NH₄Cl): A colorless, water-soluble salt commonly used as a fertilizer and food additive.
- Ammonium Nitrate (NH₄NO₃): A white, crystalline compound widely employed as a fertilizer and explosive in construction and mining.
- Ammonium Phosphate ((NH₄)₃PO₄): A water-soluble salt used extensively as a fertilizer in agriculture.
- Ammonium Bicarbonate (NH₄HCO₃): A white, crystalline powder used as a leavening agent in baking and a component in fire extinguishers.
The ammonium ion is a versatile and essential component of numerous nitrogen compounds, enriching our daily lives in countless ways. From fertilizers that nourish crops to explosives that shape our world, the ammonium ion’s influence is undeniable. Understanding its structure, properties, and common compounds empowers us to appreciate its remarkable contributions to our world.
The Nitrate Ion (NO₃⁻): A Versatile Nitrogen Compound
In the realm of ionic compounds, the nitrate ion (NO₃⁻) stands out as a ubiquitous and multifaceted player. Its triangular structure, featuring a central nitrogen atom bonded to three oxygen atoms, grants it a negative charge. This negative charge originates from the uneven distribution of electrons, with the nitrogen atom bearing a positive charge and the oxygen atoms each carrying a negative charge.
The nitrate ion forms when nitric acid (HNO₃), a strong acid, dissociates in water. In this process, the hydrogen atom donates a proton (H⁺), leaving behind the nitrate ion. Its versatility stems from its ability to combine with various positively charged ions, forming a diverse range of ionic compounds.
Among the common nitrogen compounds containing nitrate ions are:
- Ammonium nitrate (NH₄NO₃): A fertilizer used to enhance plant growth.
- Potassium nitrate (KNO₃): A component of gunpowder and fertilizers.
- Sodium nitrate (NaNO₃): A fertilizer and food preservative.
- Calcium nitrate (Ca(NO₃)₂) : A fertilizer and nutrient supplement.
The nitrate ion plays a crucial role in various biological processes, including the nitrogen cycle, which ensures the availability of nitrogen for plant growth. Despite its importance, excessive nitrate intake can pose health risks, particularly for infants and individuals with impaired kidney function.
Determining the Formula of an Ionic Compound: Balancing Charges for a Neutral Compound
When it comes to ionic compounds, determining their formula is crucial for understanding their properties and reactivity. Ionic compounds are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). To achieve a neutral compound, the total positive charge of the cations must equal the total negative charge of the anions.
Rules for Determining Ion Charges
- Metals typically form cations by losing one or more electrons, resulting in a positive charge. The number of electrons lost corresponds to the metal’s group number on the periodic table.
- Non-metals typically form anions by gaining one or more electrons, resulting in a negative charge. The number of electrons gained corresponds to the non-metal’s group number minus eight.
- Polyatomic ions (ions composed of multiple atoms) have charges determined by the net charge of the atoms they contain.
Balancing Charges
To ensure a neutral compound, the _total positive charge of the cations must equal the total negative charge of the anions. This can be achieved by adjusting the number of each type of ion present.
- Calculate the charges of the ions involved in the compound using the rules described above.
- Determine the ratio of cations to anions that will balance the charges. This ratio will typically be in the simplest whole number ratio.
- Multiply the formula of each ion by the appropriate ratio to ensure the charges balance.
Example: Determining the Formula of Ammonium Nitrate
Ammonium ion (NH₄⁺): Positive charge of _+1 (four nitrogen atoms with a total of four positive charges, balanced by one negative charge from the hydrogen atom).
Nitrate ion (NO₃⁻): Negative charge of _-1 (one nitrogen atom with a positive charge of +5, balanced by three oxygen atoms with a total of three negative charges of 6).
Balancing the charges: To achieve a neutral compound, one ammonium ion with a _+1 charge must be combined with one nitrate ion with a -1 charge._
Therefore, the formula for ammonium nitrate is NH₄NO₃.
Determining the Formula of Ammonium Nitrate (NH₄NO₃)
In the intricate world of ionic compounds, understanding their formulas is paramount. These compounds comprise two distinct entities: cations (positively charged ions) and anions (negatively charged ions).
To determine the formula of an ionic compound, we need to calculate the charges of its ions and balance them to achieve a neutral compound.
Let’s take the example of ammonium nitrate (NH₄NO₃). It consists of two ions: the ammonium ion (NH₄⁺) and the nitrate ion (NO₃⁻).
Calculating the Charges:
-
Ammonium ion (NH₄⁺): The nitrogen atom has a charge of +1 due to the loss of three electrons. The four hydrogen atoms surrounding it contribute an additional +1 charge each. Thus, the overall charge of the ammonium ion is +1.
-
Nitrate ion (NO₃⁻): The nitrogen atom has a charge of +5. However, the three oxygen atoms surrounding it contribute a total of -6 charges due to their high electronegativity. This results in an overall charge of -1 for the nitrate ion.
Balancing the Charges:
To create a neutral compound, the total positive charge must equal the total negative charge. Since the ammonium ion has a charge of +1 and the nitrate ion has a charge of -1, we need one ammonium ion for every one nitrate ion.
Therefore, the formula of ammonium nitrate is NH₄NO₃. The subscripts indicate the ratio of the ions in the compound.