Iodine: A Versatile Halogen With Antiseptic And Endocrine Significance
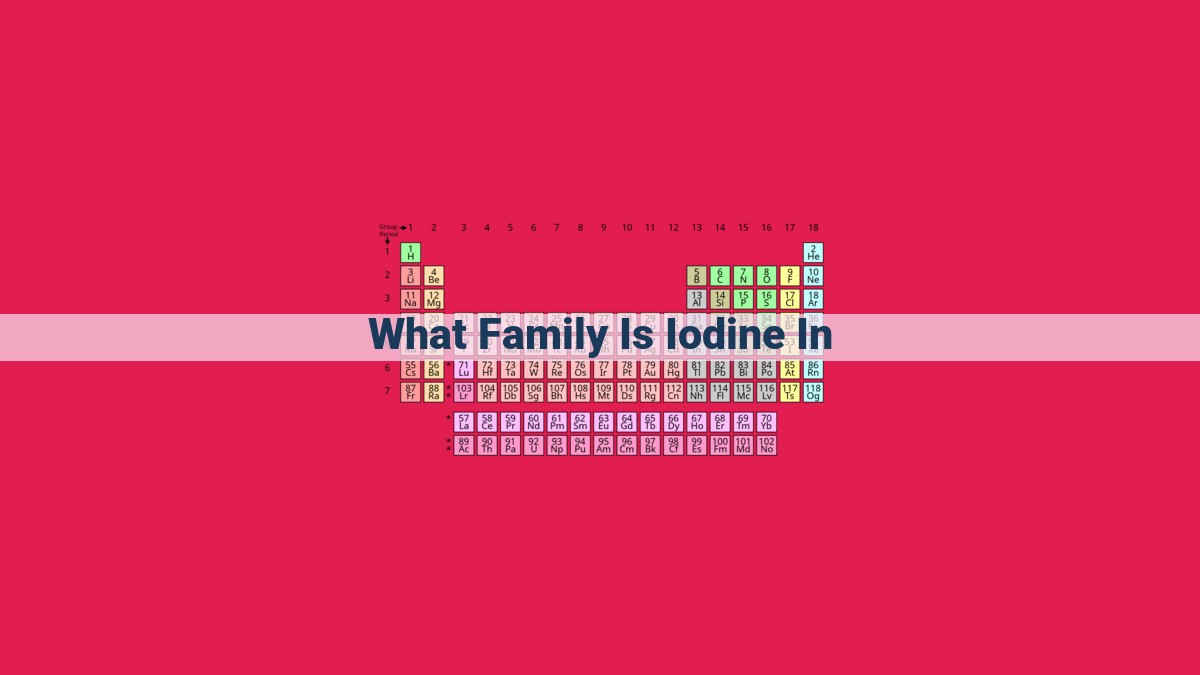
Iodine belongs to the halogen family, a group of highly reactive elements that exhibit similar chemical properties. This family includes fluorine, chlorine, bromine, iodine, astatine, and tennessine. Halogens share traits such as high electronegativity, diatomic nature, and a tendency to form salts. Iodine’s unique properties, including its antiseptic qualities and role in thyroid hormone production, make it a vital element in various fields. As part of the halogen family, iodine contributes to the diverse applications of these elements in science and industry.
The Enigmatic Halogens: Unveiling the Reactive Nature of Elements
In the realm of chemistry, there exists a family of elements known as the halogens, possessing an alluring combination of properties that sets them apart from their counterparts. These elements, namely fluorine, chlorine, bromine, iodine, astatine, and tennessine, share a common thread that defines their very essence.
Halogens: A Definition
Halogens derive their name from the Greek words “hals” (salt) and “genes” (forming), reflecting their propensity to form salts when combined with metals. They reside in Group 17 of the periodic table, characterized by their possession of seven valence electrons. This unique electronic configuration grants them an insatiable hunger for electrons, driving their high reactivity.
Elemental Characteristics of Halogens
As elemental substances, halogens exhibit distinct traits that contribute to their captivating nature. They exist as diatomic molecules, meaning they consist of pairs of atoms, denoted by the chemical symbols F2, Cl2, Br2, I2, At2, and Ts2. This molecular structure, coupled with their low melting and boiling points, renders them highly volatile substances.
Furthermore, halogens are renowned for their electronegativity, a measure of their ability to attract electrons. This characteristic, coupled with their small atomic radii, empowers them to readily form covalent bonds with other elements. Their reactivity extends to metals, forming ionic compounds, and even to nonmetals, creating covalent compounds.
In essence, the halogens are a family of elements united by their exceptional reactivity, a direct consequence of their electronic configuration and molecular structure. Their presence in various scientific and industrial processes underscores their indispensable role in our world.
Meet the Halogen Family
- List the halogen elements: fluorine, chlorine, bromine, iodine, astatine, and tennessine.
Meet the Halogen Family: A Quirky Bunch of Elements
In the periodic table’s Group 17, we find a family of elements so reactive that they can make you dance like a puppet: the halogens. These enigmatic elements share a unique bond, like mischievous siblings with a secret language. Let’s introduce the cast of characters:
Fluorine: The oldest sibling, the most electronegative (think: greedy for electrons) of the bunch. It’s so reactive that it can make even the strongest metals blush.
Chlorine: A versatile middle child, known for its role as a disinfectant and a key ingredient in table salt. It’s the element that makes swimming pools sparkle.
Bromine: The laid-back sibling, existing as a liquid at room temperature. It adds a reddish-brown hue to the Dead Sea and is used as a disinfectant.
Iodine: The multifaceted artist of the family. It’s essential for thyroid hormone production, its presence in seafood gives sushi its unique flavor, and it’s a common antiseptic.
Astatine: The mysterious and radioactive youngest sibling. It’s so rare that it’s only found in trace amounts in nature, making it a scientific enigma.
Tennessine: The newest addition to the family, discovered in 2010. It’s a superheavy element that exists only in laboratories and is named after the state of Tennessee.
These halogen siblings share a diatomic nature, meaning they form pairs of atoms to stay stable. Their high reactivity comes from their eagerness to complete their outermost electron shell, leading them to readily form bonds with other elements.
Stay tuned to discover more about the properties of halogens and how they shape our world, from medicine to industry.
Unveiling the Common Threads: Properties of Halogens
In the captivating realm of chemistry, the halogen family stands out as a group of elements that share a remarkable array of properties. These properties, akin to invisible threads, unify the halogens and define their unique behavior.
High Reactivity:
Halogens are highly reactive elements. Their strong desire to bond with other elements drives their interactions. They readily form compounds with various substances, including metals, non-metals, and even other halogens. This reactivity stems from their unfilled valence shells, making them eager to gain electrons and complete their electronic configuration.
Diatomic Nature:
In their natural state, halogens exist as diatomic molecules. This means that they form pairs of atoms, such as F2, Cl2, Br2, and I2. These diatomic molecules are held together by covalent bonds, where the atoms share electrons. The diatomic nature of halogens contributes to their high reactivity, as it allows them to break apart and form bonds with other elements.
Electronegativity:
Electronegativity measures an element’s ability to attract electrons toward itself. Halogens possess high electronegativity, meaning they have a strong tendency to pull electrons from other atoms. This property makes them highly oxidizing, enabling them to accept electrons from other species and undergo chemical reactions.
Iodine: A Spotlight on a Versatile Element
In the realm of halogens, iodine stands out with its unique properties and far-reaching applications. This essential element has played a pivotal role in human health and scientific advancements.
Properties of Iodine
Iodine ranks as the fourth lightest halogen and boasts a deep violet coloration. It is a solid at room temperature and sublimates easily when heated, forming diatomic molecules (I₂). Its electronegativity makes it a strong oxidizing agent.
Iodine in Antiseptics
One of iodine’s most notable uses is in antiseptics. Its disinfecting properties have been recognized for centuries. When applied to the skin, iodine kills bacteria and fungi, preventing infections. Tincture of iodine, a solution of iodine in alcohol, is a common first-aid antiseptic.
Iodine in Thyroid Function
Iodine is essential for the production of thyroid hormones. These hormones regulate metabolism, growth, and development. Iodine deficiency can lead to hypothyroidism, a condition marked by fatigue, weight gain, and impaired cognitive function.
Other Applications of Iodine
Beyond its medical applications, iodine is also used in various industries:
- Photography: Silver iodide is used to produce photographic plates.
- Agriculture: Iodine is added to fertilizers to prevent iodine deficiency in crops.
- Chemical Analysis: Iodine is used as an indicator in titrations.
Iodine epitomizes the diverse and essential nature of the halogen family. From its antiseptic properties that protect our health to its role in thyroid hormone production, iodine underscores the importance of this fascinating group of elements in our world.
Halogens: Essential Elements Shaping Our World
In the realm of chemistry, halogens stand as a captivating family of elements that play crucial roles in our world. Their unique properties and their presence in various compounds make them indispensable in a wide range of scientific and industrial applications.
Fluorine, the most reactive among the halogens, serves as a key ingredient in the production of fluoride toothpaste and refrigerants. Its exceptional reactivity also makes it an effective cleaning agent in the semiconductor industry.
Chlorine is perhaps the most well-known halogen thanks to its role as a disinfectant and sanitizer. It effectively neutralizes harmful microorganisms in water, swimming pools, and various surfaces. Additionally, chlorine is used in the production of PVC (polyvinyl chloride), a versatile plastic material with uses ranging from pipes to consumer products.
Bromine often finds application in the production of flame retardants and sedatives. Its presence in these compounds helps prevent the spread of fires and alleviate anxiety, respectively.
Iodine, a somewhat less common halogen, plays a vital role in the human body as a component of thyroid hormones. These hormones regulate metabolism and growth. Iodine is also used in the production of antiseptics and contrast agents used in medical imaging.
Astatine and tennessine are the rarest halogens and have limited practical applications due to their scarcity. However, their unique properties make them valuable subjects for scientific research.
In conclusion, halogens, with their diverse properties and extensive applications, are essential elements that shape our world. From disinfecting our water to powering our industries, these elements play a crucial role in maintaining our health, safety, and technological advancements.