Intermolecular Forces: Understanding The Physical Properties Of Compounds
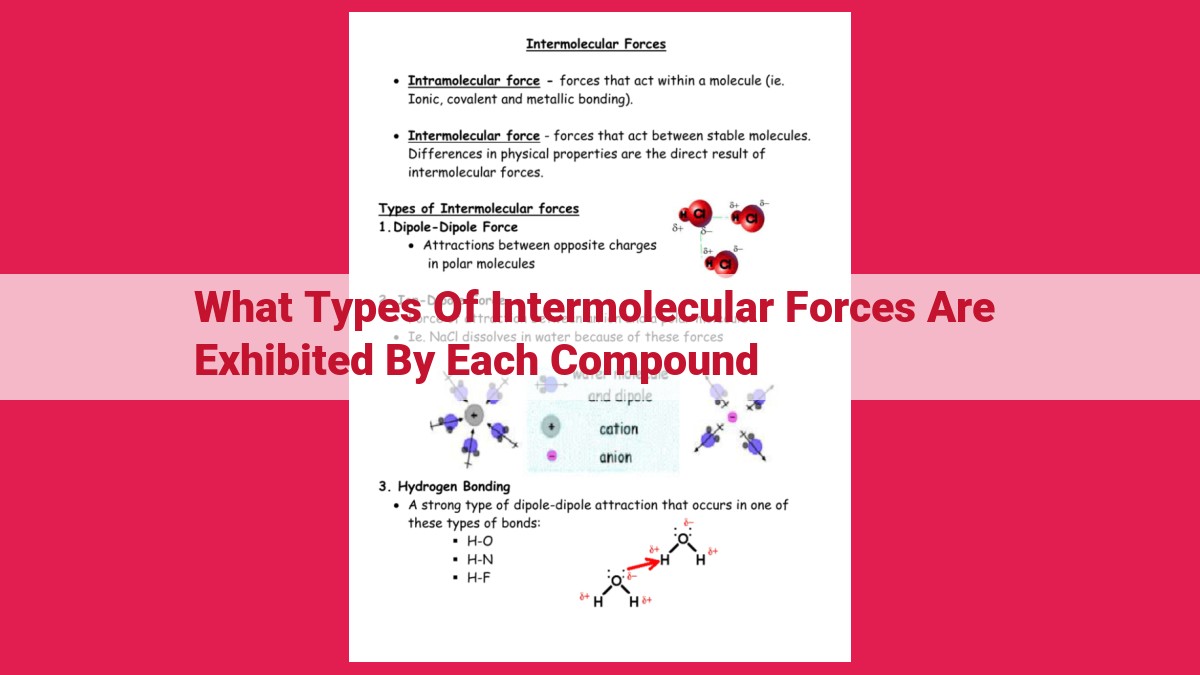
Intermolecular forces, including dipole-dipole forces, hydrogen bonding, and Van der Waals forces, determine the physical properties of compounds. Dipole-dipole forces arise from permanent dipole moments, while hydrogen bonding is a specific type of dipole-dipole force that occurs between molecules with hydrogen-electronegative atom bonds. Van der Waals forces are weak attractive forces present in all molecules due to instantaneous polarization. The strength of these forces varies, with hydrogen bonding being the strongest and Van der Waals forces being the weakest.
Intermolecular Forces: The Invisible Bonds Shaping Our World
Intermolecular forces, the subtle attractions and repulsions between molecules, are the unsung heroes of our everyday world. They determine how substances behave, influencing their physical properties such as melting point, boiling point, and solubility.
In the molecular realm, these forces are like celestial bodies, forming intricate dance patterns that orchestrate the behavior of chemical structures. Understanding these invisible bonds is like deciphering a hidden language, unlocking the secrets to why substances behave the way they do.
Dipole-Dipole Forces: The Dance of Polar Molecules
Imagine a world where molecules are not just neutral blobs, but possess a hidden dance, influenced by the delicate balance of their electrical charges. This dance is orchestrated by a force known as dipole-dipole forces, a crucial player in determining the behavior and properties of matter.
Polarity: The Birth of Dipole Moments
Molecules become polar when one end carries a slight positive charge, while the other carries a slight negative charge. This charge separation, known as polarity, arises when one atom or functional group within a molecule exerts a stronger pull on shared electrons, creating an asymmetrical distribution of charge.
The Permanent Dipoles: A Magnetic Appeal
Polar molecules possess permanent dipole moments, which represent the magnitude and direction of their charge separation. The stronger the dipole moment, the greater the polarity of the molecule. This dipole moment acts like a tiny magnet, causing polar molecules to align and attract each other.
Dance Partners: Examples of Dipole-Dipole Interactions
Compounds that exhibit dipole-dipole forces include polar solvents like water (H2O), ethanol (C2H5OH), and acetone (CH3COCH3). These molecules have a net dipole moment due to the electronegative nature of oxygen atoms, creating a partial negative charge on one end of the molecule.
Water, a prime example of a polar solvent, owes its unique properties to dipole-dipole forces. The attraction between water molecules is responsible for water’s high boiling point and high surface tension, enabling it to form droplets and dissolve ionic compounds. In contrast, nonpolar molecules like hexane (C6H14), which lack a net dipole moment, interact primarily through weak Van der Waals forces, resulting in lower boiling points.
The Impact of Polarity: Unraveling the Dance
The strength of dipole-dipole forces depends on the size and shape of the molecule, as well as the magnitude of its dipole moment. Molecules with larger dipole moments experience stronger dipole-dipole forces, leading to increased molecular cohesion and higher melting and boiling points.
Dipole-dipole forces play a vital role in determining the solubility of substances. Polar solvents, such as water, readily dissolve polar solutes, as the dipole-dipole interactions between the solvent and solute molecules overcome the solute’s cohesive forces. On the other hand, nonpolar solvents are poor solvents for polar solutes due to weak or absent dipole-dipole interactions.
Hydrogen Bonding: An Extraordinary Intermolecular Force
In the world of chemistry, molecules often engage in captivating relationships called intermolecular forces. Among these forces, hydrogen bonding stands out as an exquisite and impactful dance between molecules.
Defining Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole force that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This creates a polar covalent bond with a significant difference in electronegativity between the hydrogen and the other atom.
Distinctive Feature: Hydrogen’s Role
What makes hydrogen bonding unique is the hydrogen’s ability to participate in multiple bonds. The hydrogen atom, with its lone electron, can form an additional bond with another electronegative atom, creating a partial positive charge on the hydrogen. This partial positive charge then interacts with the partial negative charge on the neighboring electronegative atom, resulting in an electrostatic attraction.
Strength and Significance
Hydrogen bonding is significantly stronger than typical dipole-dipole forces. This is because the electrostatic attraction between the partial charges is enhanced by the small size and high electronegativity of hydrogen. Hydrogen bonding plays a crucial role in determining the physical properties of many substances, influencing their melting and boiling points, solubility, and stability.
Examples of Hydrogen-Bonded Compounds
Hydrogen bonding is prevalent in a diverse range of compounds, including:
- Water (H2O): The classic example of hydrogen bonding, responsible for water’s high boiling point, cohesion, and solvent properties.
- Ammonia (NH3): Hydrogen bonding between ammonia molecules contributes to its low boiling point and high solubility in water.
- DNA: The double helix structure of DNA is stabilized by hydrogen bonds between base pairs, ensuring genetic information’s integrity.
- Proteins: Hydrogen bonding within protein chains plays a vital role in protein folding and function.
Van der Waals Forces: The Glue That Holds Molecules Together
In the fascinating world of chemistry, molecules are not isolated entities; they interact with each other through a multitude of forces. Among these forces, Van der Waals forces play a crucial role in determining the physical properties of substances and influencing chemical reactions.
Defining Van der Waals Forces
Imagine that molecules are like tiny playgrounds where electrons dance around the atomic nuclei. These electrons are constantly in motion, creating brief, fleeting moments where they are unevenly distributed within a molecule. This phenomenon is known as instantaneous polarization.
As molecules with instantaneously polarized regions approach each other, they experience a weak attractive force. These attractive forces, known as Van der Waals forces, are named after the Dutch scientist Johannes Diderik van der Waals, who first proposed their existence.
Types of Van der Waals Forces
There are three primary types of Van der Waals forces:
-
Dipole-induced dipole forces: When a polar molecule comes close to a nonpolar molecule, it can induce a dipole in the nonpolar molecule. This results in an attractive force between the polar molecule and the induced dipole.
-
Dipole-dipole induced forces: When a polar molecule comes close to another polar molecule, the electric field of the first molecule can induce a dipole in the second molecule. This leads to an attractive force between the two polar molecules.
-
London dispersion forces: These forces are present in all molecules, regardless of their polarity. They arise from the instantaneous polarization and temporary fluctuations in electron distribution within molecules.
Impact on Properties of Substances
Van der Waals forces play a significant role in determining the physical properties of substances. They influence factors such as:
- Melting point: Substances with stronger Van der Waals forces require more energy to overcome the attractive forces between molecules, resulting in a higher melting point.
- Boiling point: Similarly, stronger Van der Waals forces lead to a higher boiling point, as more energy is needed to break the intermolecular bonds and convert the liquid to a gas.
- Solubility: Van der Waals forces can affect the solubility of a substance in a solvent. Generally, substances with similar intermolecular forces have greater solubility in each other.
Examples of Van der Waals Forces
Van der Waals forces are ubiquitous in chemistry. Some notable examples include:
- Noble gases: These gases consist of nonpolar molecules and primarily interact through London dispersion forces.
- Alkanes: These nonpolar hydrocarbons have strong Van der Waals forces due to their low polarity and large molecular size.
- Water: Despite its polarity, water also experiences London dispersion forces between its molecules. These forces contribute to the cohesive properties of water.
Importance in Applications
The understanding of Van der Waals forces has wide-ranging applications in various scientific fields, including:
- Drug design: By manipulating Van der Waals forces, scientists can tailor the interactions between drug molecules and biological targets.
- Materials science: Control over Van der Waals forces enables the design of materials with enhanced properties, such as improved strength, flexibility, and conductivity.
- Nanotechnology: Van der Waals forces are exploited in the assembly and manipulation of nanostructures and devices.
Van der Waals forces are essential intermolecular forces that influence a vast array of chemical phenomena. By understanding the nature and strength of these forces, scientists can unravel the behavior of molecules, predict physical properties, and design materials and applications with tailored properties.
Summary and Impact
- Summarize the different types of intermolecular forces and their strengths.
- Explain how these forces affect the behavior and properties of compounds.
- Discuss the importance of intermolecular forces in various physical processes and applications.
Understanding Intermolecular Forces and Their Influence on Compounds
In the realm of chemistry, molecules don’t exist in isolation. They interact with each other through intermolecular forces, which play a crucial role in determining their physical properties and behavior.
Intermolecular forces are like the invisible threads that knit molecules together, influencing their melting and boiling points, solubility, and many other aspects. These forces can be strong (like hydrogen bonds) or weak (like Van der Waals forces), and their strength governs the cohesion and behavior of the compounds they hold together.
The Spectrum of Intermolecular Forces
There are three main types of intermolecular forces, each with its own unique characteristics and strengths:
1. Dipole-Dipole Forces:
When molecules have a permanent imbalance in their electron distribution, they acquire a polarity. This polarity creates permanent dipole moments, which can interact with each other through attractive forces. The stronger the dipole moment, the stronger the dipole-dipole forces.
2. Hydrogen Bonding:
A special case of dipole-dipole forces, hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen or nitrogen). This creates a strong electrostatic attraction, resulting in the formation of hydrogen bonds. Hydrogen bonding is exceptionally strong and plays a critical role in many biological systems.
3. Van der Waals Forces:
Present in all molecules, Van der Waals forces arise from instantaneous polarizations. Induced dipoles in one molecule can attract neighboring molecules, resulting in weak but additive forces. These forces are generally weaker than dipole-dipole and hydrogen bonding interactions.
Impact on Compound Behavior and Properties
Intermolecular forces have a profound impact on the properties of compounds:
- Strong forces, such as hydrogen bonds, lead to higher melting and boiling points due to the increased energy required to overcome these bonds.
- Weak forces, such as Van der Waals forces, result in lower melting and boiling points.
- Solubility is influenced by intermolecular forces. Compounds with similar intermolecular forces (e.g., polar solvents with polar solutes) tend to be more soluble in each other.
Applications and Importance
The importance of intermolecular forces extends beyond their influence on compound properties:
- They play a crucial role in biological systems, such as the structure of DNA and the interaction between proteins.
- In materials science, engineers manipulate intermolecular forces to design materials with specific properties.
- Pharmacology relies on intermolecular forces to understand the interactions between drugs and biological molecules.
By understanding the different types of intermolecular forces and their strengths, scientists and engineers can tailor the properties of compounds for a wide range of applications.