Indium: Unveiling The Valence Electron Count And Its Chemical Significance
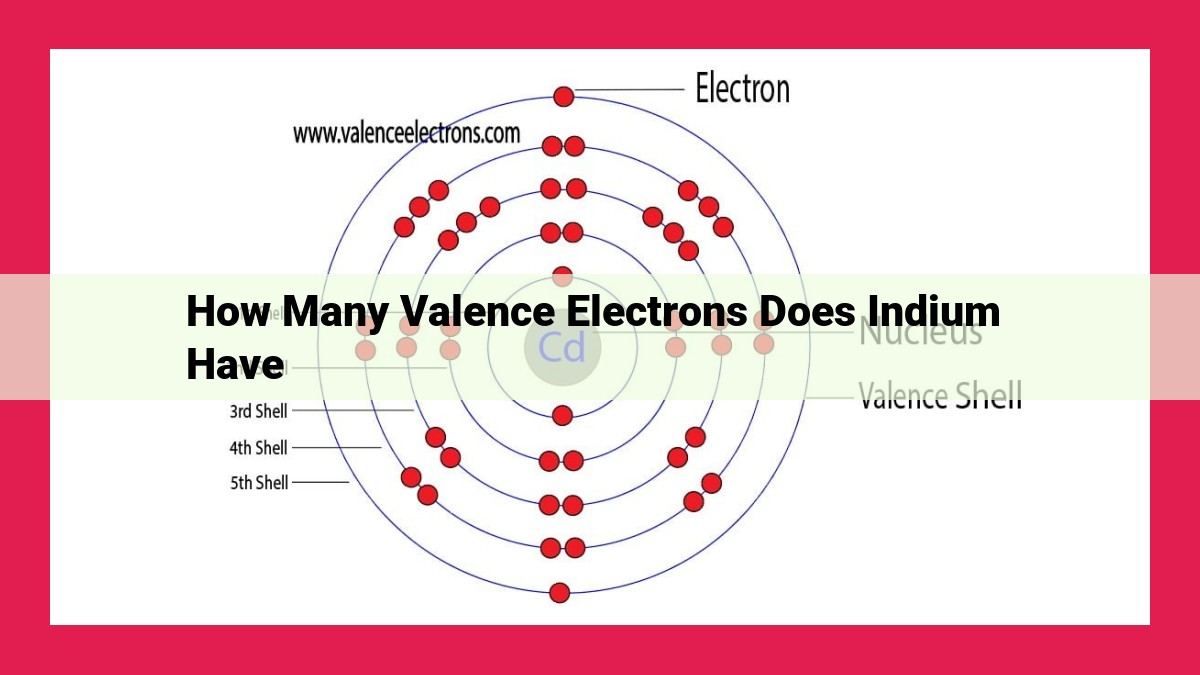
How Many Valence Electrons Does Indium Have?
Indium, an element in Group 13 of the periodic table, possesses a unique electron configuration that grants it specific chemical properties. Focusing on its valence electrons, which dictate an element’s bonding behavior, we delve into the atomic structure of indium. With an electron configuration of [Kr]4d¹⁰4p¹, indium has a single valence electron, residing in the 4p orbital. This lone valence electron enables indium to form various chemical bonds, such as covalent, ionic, and metallic, depending on the bonding circumstances.
Understanding Valence Electrons: Unveiling the Secrets of Chemical Bonding and Reactivity
In the realm of chemistry, the concept of valence electrons holds immense significance in determining the behavior and interactions of atoms. Valence electrons are the electrons that occupy the outermost energy level of an atom, playing a crucial role in chemical bonding and the element’s overall reactivity.
One intriguing element that exemplifies the importance of valence electrons is Indium. This silvery-white metal has captured the attention of scientists due to its unique properties and wide range of applications. Understanding the number of valence electrons indium possesses is essential to unraveling its intriguing chemical nature.
Embark on a captivating journey into the world of valence electrons as we delve into the question: How many valence electrons does indium have?
Electron Configuration of Indium
- Describe the electronic configuration of indium (1s22s22p63s23p64s23d104p1).
- Highlight the valence electrons (4p1).
Indium’s Electron Configuration and Its Significance
Let’s dive into the fascinating world of chemistry and explore the importance of understanding an element’s electron configuration. Today, we’re going to focus on indium, a metal with a unique property that gives it a wide range of applications.
Electronic Configuration of Indium
Indium, with the atomic number 49, has an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p¹. This notation tells us how electrons are distributed in specific energy levels, called atomic orbitals.
The valence electrons are the electrons in the outermost energy level, which are responsible for an element’s chemical properties and reactivity. For indium, it has one valence electron in the 4p orbital.
Valence Electrons and Atomic Orbitals
Remember that electrons like to hang out in specific orbitals, which represent different shapes and energy levels. The four types of orbitals are designated as s, p, d, and f. Valence electrons occupy the p-orbitals, which have three lobes, one of which points towards the nucleus and two that point away.
In the case of indium, its single valence electron resides in the 4p orbital, which has a dumbbell shape with one lobe pointing towards and one away from the nucleus. This unique electron arrangement gives indium its distinctive chemical properties.
Chemical Bonding and Valence Electrons
Valence electrons play a crucial role in determining how elements form chemical bonds. Indium’s single valence electron allows it to participate in various types of bonding.
- Covalent Bonding: Indium can share its valence electron with another atom, forming a covalent bond.
- Ionic Bonding: Indium can lose its valence electron, forming a positive ion that can bond with a negatively charged ion.
- Metallic Bonding: Indium can also form metallic bonds, where its valence electrons are shared among a group of metal atoms.
Indium on the Periodic Table
Indium is located in Group 13 (also known as the Boron group) and Period 5 of the periodic table. Elements in the same group have similar valence electron configurations, which gives them similar chemical properties.
Periodic Trends and Group 13 Elements
The periodic table is a powerful tool that can help us understand an element’s properties based on its position. Indium’s properties follow certain periodic trends:
- Metallic Character: Elements in Group 13 exhibit metallic character, meaning they are good conductors of heat and electricity.
- Reactivity: Indium is less reactive than other Group 13 elements, such as aluminum and gallium, due to the presence of its filled 3d orbitals.
The Significance of Valence Electrons
Understanding valence electrons is crucial for predicting an element’s reactivity and bonding capabilities. Indium, with its single valence electron, has unique chemical properties that make it useful in a variety of applications, including:
- Semiconductors in electronic devices
- Alloys for soft solder and bearings
- Coatings to protect metals from corrosion
- Catalysts in chemical reactions
By exploring the electron configuration of indium, we gain a deeper appreciation for the role of valence electrons in shaping the chemical world around us.
Valence Electrons and Atomic Orbitals
To truly understand the captivating dance of chemical bonding and reactivity, we must unveil the secrets of valence electrons. As we embark on this journey, let’s focus our lens on indium, an element with a unique story to tell.
Indium’s Electronic Haven
Indium’s electric blueprint, known as its electron configuration, is written as 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p¹. This string of numbers and letters reveals the energy levels and the number of electrons occupying each level. The key players in our story are the valence electrons, the outermost electrons that roam the highest energy level, the 4p orbital. In indium’s case, it has a solitary valence electron, eagerly waiting to engage in the intriguing world of chemical bonding.
Orbiting around the Nucleus
Electrons don’t just float around aimlessly; they occupy specific orbitals, which are regions of space where they are most likely to be found. These orbitals have specific shapes and orientations, and the valence electrons reside in the 4p orbital, which is a dumbbell-shaped region extending in three dimensions. This orbital diagram of indium depicts the lone valence electron, like a star shining in the vastness of the 4p orbital.
The Dance of Bonding
Valence electrons play a pivotal role in the thrilling world of chemical bonding. They are the keys that unlock the ability of atoms to form partnerships, creating molecules and compounds. Indium’s single valence electron makes it a versatile dance partner, allowing it to participate in a variety of bonds. It can form covalent bonds, sharing electrons with other atoms, ionic bonds, transferring electrons to become positively charged, or even metallic bonds, forming a sea of electrons that holds metal atoms together.
Indium’s position in the periodic table, Group 13 and Period 5, further sheds light on its electron configuration and valence electron count. Group 13 elements share the same number of valence electrons, giving them similar chemical properties. Indium’s neighbors, gallium and thallium, also have one valence electron, hinting at the cohesive nature of valence electrons within a group.
We’ve explored the captivating world of valence electrons, their cozy abode in atomic orbitals, and their pivotal role in chemical bonding. Indium, with its single valence electron, serves as a shining example of how these elusive particles shape the chemical landscape around us. Understanding valence electrons empowers us to predict reactivity, bonding capabilities, and the diverse behaviors of elements in the periodic table.
Chemical Bonding and Valence Electrons: The Key to Understanding Indium’s Reactivity
The intricate world of chemistry revolves around the interactions between atoms, and valence electrons play a pivotal role in this dance. These electrons, residing in the outermost shell of an atom, determine its ability to form chemical bonds and dictate its chemical reactivity.
Indium’s Single Valence Electron: A Versatile Bonding Partner
Indium, an element nestled in Group 13 of the periodic table, possesses a unique characteristic: it has only one valence electron. This lone electron, occupying the 4p orbital, endows indium with an unparalleled bond-forming prowess.
Covalent Bonding: Sharing the Lone Electron
In the realm of covalent bonding, atoms join hands by sharing their valence electrons. Indium’s single valence electron eagerly participates in this cooperative dance, forming strong covalent bonds with nonmetals like chlorine or oxygen, resulting in compounds such as indium chloride (InCl3) and indium oxide (In2O3).
Ionic Bonding: Transfer of the Lone Electron
Sometimes, indium prefers a more dramatic relationship, engaging in ionic bonding. In this scenario, indium donates its lone valence electron to a more electronegative partner like fluorine. This electron transfer creates positively charged indium ions (In3+) and negatively charged fluorine ions (F-), which are held together by electrostatic forces. Indium fluoride (InF3) is a classic example of an ionic compound.
Metallic Bonding: A Sea of Electrons
When indium encounters its own kind, it forms metallic bonds, characterized by a “sea” of delocalized electrons. These electrons are not confined to individual atoms but roam freely throughout the metal lattice, giving indium its malleability, ductility, and ability to conduct electricity.
Indium’s single valence electron serves as a catalyst for its diverse bonding capabilities, enabling it to form covalent, ionic, and metallic bonds. Understanding the role of valence electrons is not just a matter of scientific curiosity but a key to unraveling the intricate tapestry of chemical reactions and predicting the reactivity of elements.
Indium on the Periodic Table
- Define the concept of elements and the periodic table.
- Describe indium’s position in Group 13 and Period 5.
Indium’s Position on the Periodic Table
In the realm of chemistry, understanding the characteristics of elements is crucial for unraveling their reactivity and bonding abilities. The periodic table serves as a roadmap, organizing elements based on their properties and atomic structure. Indium, our element of interest, finds its place in Group 13 and Period 5.
Elements and the Periodic Table
Elements are the fundamental building blocks of matter, each possessing a unique atomic number that distinguishes it from all others. The periodic table is a tabular arrangement of elements, organized according to their atomic numbers, electron configurations, and recurring chemical properties.
Indium’s Position in the Periodic Table
Indium, an element symbolized by In, resides within the 13th group of the periodic table, also known as the boron group. This group comprises elements that share three valence electrons, playing a crucial role in their chemical behavior. Furthermore, indium occupies the 5th period, indicating that its atoms possess five energy levels.
Indium’s position on the periodic table provides valuable insights into its properties. Elements within the same group tend to exhibit similar chemical characteristics, allowing us to draw inferences about indium based on its group membership. Moreover, its position in Period 5 suggests that indium is a transition metal with a relatively large atomic size and diverse bonding capabilities.
Periodic Trends and Group 13 Elements
Indium’s Position on the Periodic Table
The periodic table is a tabular arrangement of elements organized by their atomic number, electron configuration, and recurring chemical properties. Indium, denoted by the symbol In, resides in Group 13 (also known as the Boron group) and Period 5 of the table.
Group 13 Characteristics and Similarities with Indium
Group 13 elements share several common characteristics that arise from their similar electron configurations. Their outermost electron shells contain three valence electrons. This arrangement results in a distinctive set of chemical properties:
- Trivalency: They tend to form stable compounds with a valence of three.
- Amphoteric Nature: They exhibit both acidic and basic properties, reacting with both acids and bases.
- Metallic Appearance: Due to their low electronegativity, they possess a shiny metallic luster.
- High Boiling Points: Their strong metallic bonds lead to high boiling points.
Indium’s Properties in Relation to Group 13
Indium’s position within Group 13 significantly influences its properties. Its single valence electron allows it to fulfill the trivalent bonding requirement, ensuring the formation of stable compounds. Like other Group 13 elements, indium displays:
- Metallic Appearance: With a silvery-white luster, indium exhibits the typical metallic characteristics.
- Soft and Malleable: Its low electronegativity contributes to its softness and malleability.
- High Boiling Point: The strong metallic bonds result in a high boiling point of 2,072°C (3,762°F).
Importance of Understanding Indium’s Position
Comprehending indium’s location within the periodic table and its similarities to other Group 13 elements provides valuable insights into its chemical behavior. By recognizing the periodic trends associated with its position, we can predict its reactivity, bonding capabilities, and overall chemical properties.
The Significance of Valence Electrons
- Re-emphasize the importance of valence electrons in determining an element’s chemical properties.
- Use indium as an example to illustrate how its single valence electron influences its bonding behavior.
The Significance of Valence Electrons
Valence electrons play a pivotal role in determining the chemical properties of an element. They are the outermost electrons in an atom and dictate an element’s ability to form bonds with other atoms.
Indium: A Case Study
Indium, a metal with a unique set of properties, serves as an excellent example of the significance of valence electrons. Indium has only one valence electron, located in the 4p orbital. This single electron has a profound impact on indium’s bonding capabilities.
Indium’s lone valence electron allows it to form covalent bonds, sharing electrons with other atoms to create molecules. It can also participate in ionic bonding, where it transfers its valence electron to another atom, resulting in the formation of ions. Additionally, indium’s valence electron contributes to its metallic bonding, forming a lattice structure with strong metallic bonds.
Indium’s position in Group 13 of the periodic table further highlights the importance of valence electrons. Group 13 elements all have three valence electrons, which gives them similar bonding behaviors. This periodic trend reinforces the notion that valence electrons are crucial in determining an element’s chemical properties.
In conclusion, valence electrons are of paramount importance in chemistry. They determine an element’s ability to form bonds, influence its reactivity, and shape its unique properties. Indium, with its single valence electron, exemplifies the profound impact that valence electrons have on the chemical world.