Understanding The Impact Of Thermal Energy: Temperature, Expansion, And Phase Changes
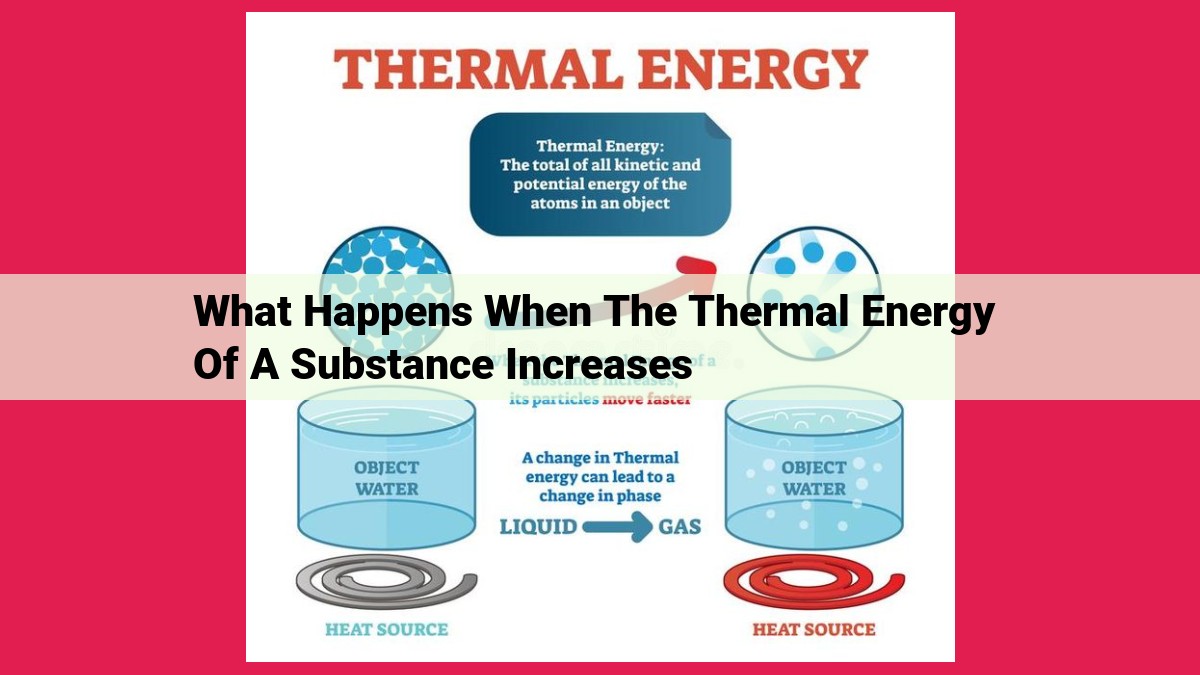
As the thermal energy of a substance increases, the molecular motion within the substance accelerates, resulting in an increase in its temperature. This increased kinetic energy leads to expansion as the molecules move further apart, increasing the substance’s volume. Depending on the temperature reached, phase changes can occur, such as melting, boiling, or sublimation. In gases, thermal energy directly influences pressure, as the increased molecular motion exerts more force on the container walls.
Thermal Energy: The Invisible Force Shaping Our World
In the tapestry of our physical surroundings, an unseen energy weaves its intricate threads, influencing the very nature of matter itself. This enigmatic force, known as thermal energy, holds the power to transform substances and shape the world around us.
Thermal energy, put simply, is the internal energy possessed by a substance due to the ceaseless motion of its molecules. Imagine a microscopic ballet, where each molecule dances with vigor, colliding and ricocheting off one another in a whirlwind of activity. The more energy these molecules possess, the faster and more chaotically they move.
This molecular motion is not merely a spectator sport; it has profound implications for the physical properties of substances. As thermal energy increases, so too does the velocity of the molecules, leading to a plethora of fascinating phenomena. Understanding the relationship between thermal energy and molecular motion is the key to unlocking the secrets of matter and its remarkable transformations.
Molecular Motion: The Dance of Thermal Energy
As we ignite thermal energy within substances, we witness a surge of activity at the molecular level. Imagine tiny dancers confined within a room, their movements restricted. But as we turn up the thermal dial, the room expands, granting them more space to frolic. Their energy levels skyrocket, propelling them into a frenzy of motion.
This molecular ballet is intimately connected to temperature, a measure of the average kinetic energy of these tiny dancers. As their energy intensifies, so does the temperature, making them velocity demons. This explains why substances heat up as they absorb thermal energy, their molecules accelerating and colliding with greater force.
Temperature Increase: The Dance of Thermal Energy
Embark on a Journey into the Realm of Heat
In the vibrant world of science, thermal energy reigns supreme as the driving force behind myriad transformations we witness in our daily lives. One such transformation is the captivating dance of temperature increase, where the addition of thermal energy orchestrates a harmonious symphony of molecular motion.
The Essence of Temperature
At its core, temperature is an intrinsic property that quantifies the average kinetic energy of molecules within a substance. Kinetic energy, the energy of motion, embodies the frenzied dance of molecules as they traverse their microscopic realm.
Thermal Energy’s Catalytic Role
As thermal energy infuses a substance, it acts as a catalyst, accelerating the molecular motion. This accelerated dance translates into an increase in temperature. The newfound energy transforms into heat, a form of thermal energy that flows between objects of different temperatures. This heat, in turn, propels molecules to even greater heights of kinetic energy, perpetuating the cycle of temperature increase.
Comprehending the Energy Conversion
Witnessing the phenomenon of temperature increase, one cannot overlook the intricate conversion of thermal energy into kinetic energy. As thermal energy enters the molecular realm, it prompts molecules to shake and rattle with greater vigor. This heightened molecular motion manifests as an increase in temperature, and the substance’s internal energy escalates.
By delving into the transformative power of thermal energy, we uncover the profound significance of temperature increase and its indispensable role in shaping the behavior of matter.
Expansion: How Thermal Energy Makes Substances Grow
As we crank up the thermal energy within substances, they begin to expand, meaning their volume increases. Imagine a balloon filled with air. As the temperature inside the balloon rises, the air particles start buzzing around even faster, colliding with each other and the balloon’s walls more frequently. This relentless bombardment pushes the balloon’s walls outward, making it expand.
The same principle applies to all substances, solid, liquid, or gas. The more thermal energy they absorb, the more energetic their molecules become, causing them to move and collide more often. This increased activity translates into greater volume as the molecules push against each other and the container’s walls.
Now, let’s talk about density. Density is a measure of how much mass a substance packs into a given volume. As a substance expands, its volume increases, while its mass remains constant. This means that the density of the substance decreases.
Phase changes also play a role in expansion. When a solid melts into a liquid, the molecules become less rigidly bound and can move more freely. This leads to a noticeable volume increase, resulting in expansion. Similarly, when a liquid transforms into a gas, the molecules gain even more freedom of movement and the volume expands even further. Conversely, when a gas condenses into a liquid, or a liquid freezes into a solid, the volume decreases as the molecules slow down and pack more tightly together.
Understanding expansion is crucial for a wide range of applications. From the funcionamiento of thermometers to the design of bridges and engines, expansion plays a vital role in our everyday lives. So, remember, when you turn up the heat, you’re not just making things hotter; you’re also making them bigger!
Thermal Energy and Phase Changes: A Story of Transformation
Imagine a world where matter could effortlessly transform between different states. This phenomenon, known as phase changes, is driven by the magical force of thermal energy. Let’s embark on a captivating journey to understand the interplay between these elements.
Melting:
As thermal energy infuses a solid, its rigid molecules begin to break free. Like tiny dancers, they gain speed and momentum, disrupting the orderly structure that once held them captive. Gradually, the solid transforms into a liquid, its particles flowing freely. Imagine a solid block of ice slowly turning into a gently flowing river.
Freezing:
The reverse process unfolds as thermal energy is withdrawn from a liquid. The once-frenetic molecules slow down, forming bonds that solidify the liquid. Water transforms into ice, snowflakes crystallizing in the winter breeze.
Boiling:
When a liquid’s thermal energy reaches a critical point, a dramatic shift occurs. As bubbles of vapor burst through its surface, the liquid vaporizes into a gas. Picture a bubbling pot of water transitioning into steam, rising like an ethereal cloud.
Condensation:
As gaseous molecules lose thermal energy, they condense back into a liquid. Clouds form as water vapor in the air cools, droplets of water reappearing as rain or dew.
Thermal Energy: The Orchestrator
Thermal energy plays the pivotal role in facilitating these remarkable transformations. By adjusting the temperature of a substance, we can induce phase changes that unlock new properties and possibilities. Understanding the intricate connection between thermal energy and phase changes empowers us to harness the transformative power of nature for countless applications, from refrigeration to power generation.
Pressure Increase: How Thermal Energy Influences Gas Behavior
Imagine yourself on a scorching summer day, with the sun beating down relentlessly. The air feels thick, and you can’t help but notice how the pressure seems to have increased. What’s happening behind the scenes? It’s all about thermal energy.
As thermal energy increases, the molecular motion of gas particles also increases. These particles start moving and colliding faster, bouncing off each other and the walls of their container. As they do so, they exert a greater force on the container’s walls, resulting in an increase in pressure.
This relationship between thermal energy and pressure is beautifully explained by the Gas Laws. The Boyle-Mariotte Law states that at constant temperature, the pressure of a gas is inversely proportional to its volume. So, as thermal energy increases, the volume of the gas increases, causing a decrease in pressure.
However, another Gas Law, the Charles’s Law, states that at constant volume, the pressure of a gas is directly proportional to its temperature. This means that as thermal energy increases, the temperature of the gas also increases, leading to a further increase in pressure.
This concept of thermal energy and pressure is crucial in understanding the behavior of gases in various applications. For instance, in your car tires, the pressure increases as the air inside heats up due to friction. Similarly, in a hot air balloon, the heated air inside the balloon expands, increasing its volume and creating pressure, which lifts the balloon off the ground.
Therefore, understanding the influence of thermal energy on the pressure of gases is essential for comprehending numerous phenomena in our world, from the inflation of balloons to the operation of engines.