Impact Of Temperature On Matter: Changes In Volume, Pressure, And More
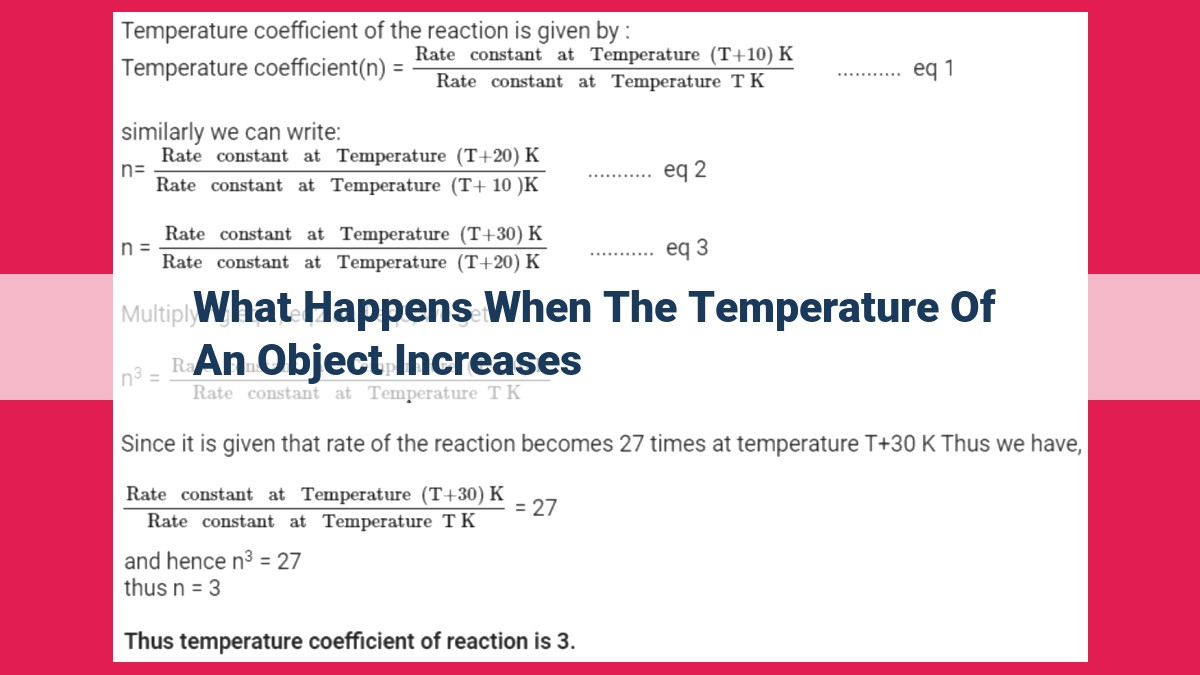
When an object’s temperature rises, its molecules gain energy, leading to increased molecular motion. This results in thermal expansion, increasing pressure, volume, and fluidity. Additionally, increased molecular kinetic energy enhances chemical reaction rates, solubility, and electrical conductivity. Thermal expansion and increased molecular motion also directly impact properties such as vapor pressure. These changes are evident in everyday phenomena like the expansion of metals and liquids with rising temperatures.
Thermal Expansion: The Dance of Molecules
In the enchanting tapestry of nature, temperature plays a captivating role, dancing with molecules to create a symphony of effects. As the temperature ascends, molecules burst into a spirited waltz, their movements accelerating in a graceful display. This energetic dance leads to a cascade of consequences, shaping the physical properties of matter and orchestrating the symphony of life.
Increased Pressure: A Force Awakens
As the molecules twirl and spin, they collide more frequently with their surroundings, exerting a greater force on the container walls. This surge in collisions translates into increased pressure, pushing against boundaries and creating the potential for transformative effects.
Expanded Volume: A Symphony of Space
With each molecule’s energetic leap, the space between them expands, creating more volume for the substance. This phenomenon manifests in the familiar expansion of materials as they heat up, whether it’s the gentle rise of a mercury thermometer or the colossal growth of a molten steel beam.
Enhanced Fluidity: A Fluidic Grace
The heightened molecular motion fosters increased fluidity, reducing the resistance to flow. Liquids become less viscous, allowing them to pour with greater ease, while gases spread and expand with remarkable agility. This fluidity is crucial for countless processes, from the flow of blood through our veins to the soaring of clouds in the sky.
Elevated Vapor Pressure: A Prelude to Evaporation
As the molecules gain energy, they become increasingly eager to break free from the liquid’s embrace. This increased vapor pressure drives the process of evaporation, where molecules escape into the air as a vapor, leaving behind a cooler, less energetic liquid.
Enhanced Solubility: A Molecular Embrace
Temperature acts as a catalyst for increased solubility, as the energetic molecules gain a greater affinity for solute particles. This entwinement of solute and solvent enhances the formation of solutions, playing a vital role in chemical reactions and biological processes.
Accelerated Chemical Reactions: A Fast-Paced Orchestra
The dance of molecules fuels increased chemical reaction rates, providing the necessary energy to overcome activation barriers. As the molecules collide with greater force and frequency, reactions proceed at an accelerated pace, shaping the chemical tapestry of our world.
Enhanced Conductivity: A Symphony of Currents
In the realm of electricity, temperature can have a profound impact on conductivity. For metals, increased temperature enhances the mobility of free electrons, facilitating the flow of current and increasing conductivity. However, in semiconductors and insulators, rising temperature can disrupt atomic and electron arrangements, decreasing conductivity.
How Temperature Affects Molecular Kinetic Energy and Its Consequences
Temperature plays a significant role in influencing the behavior of matter, affecting its molecular motion and properties. One crucial aspect of this relationship is the impact of temperature on molecular kinetic energy, which has profound consequences on various physical and chemical phenomena.
Increased Temperature, Increased Kinetic Energy
As we increase the temperature of a substance, its molecules gain energy. This energy is manifested as increased molecular kinetic energy, which refers to the energy of molecules in motion. With more energy, molecules move faster and become more energetic, leading to a cascade of effects on the substance’s properties.
Consequences of Increased Molecular Kinetic Energy
1. Increased Pressure:
* As molecules move faster, they collide with the container walls more frequently and with greater force, resulting in increased pressure exerted on the container.
2. Increased Volume:
* The faster-moving molecules require more space to move freely, causing the substance to expand in volume.
3. Increased Fluidity:
* With increased kinetic energy, the molecules overcome intermolecular forces more easily, leading to increased fluidity or decreased viscosity of the substance.
4. Increased Vapor Pressure:
* Faster-moving molecules have a higher tendency to escape the liquid or solid phase, resulting in increased vapor pressure.
5. Increased Solubility:
* Increased temperature enhances the kinetic energy of both solute and solvent molecules, allowing for better mixing and increased solubility.
6. Increased Chemical Reaction Rates:
* More energetic molecules have a higher probability of colliding and reacting with each other, leading to faster chemical reactions.
7. Increased Conductivity:
* In metals, increased temperature enhances the movement of free electrons, resulting in increased electrical conductivity.
The direct impact of increased temperature on molecular kinetic energy has far-reaching consequences on various properties of matter. By understanding these effects, we gain insights into a wide range of phenomena, from the flow of liquids to the rates of chemical reactions.
Temperature’s Impact on Electrical Conductivity
As we crank up the heat, temperature plays a fascinating role in shaping the electrical behavior of substances. Let’s dive into the intriguing ways temperature can affect electrical conductivity.
Metals: Enhanced Electron Flow
In the realm of metals, temperature is a catalyst for increased electrical conductivity. As the temperature climbs, metal atoms become more energetic, and their free electrons gain momentum. These electrons zip through the metal lattice with greater ease, facilitating the flow of electric current. This phenomenon is akin to a highway becoming less congested as the number of cars on it decreases.
Semiconductors and Insulators: A Delicate Balance
Semiconductors and insulators, on the other hand, exhibit a more complex relationship with temperature. Initially, as temperature rises, their electrical conductivity may increase. However, this increase is short-lived. At a critical temperature, the atomic or electron arrangement within these materials becomes disrupted, impairing their ability to conduct electricity. Imagine a jigsaw puzzle where the pieces are rearranged, making it harder for current to pass through.
Applications
Temperature’s influence on electrical conductivity has widespread applications. In superconductivity, for instance, certain materials exhibit zero electrical resistance at extremely low temperatures. This phenomenon is exploited in high-speed magnets and energy-efficient power transmission. Conversely, the temperature-dependent conductivity of semiconductor devices is crucial for electronic circuits and transistors.
Temperature is a potent force in the world of electrical conductivity. It can boost the flow of electrons in metals, while hindering it in semiconductors and insulators. Understanding these effects is essential for designing efficient and reliable electronic devices. So, the next time you turn on a light or use a smartphone, remember that temperature plays a hidden but vital role in making it all happen.