Identifying The Weakest Acid: Understanding Acid Strength Factors
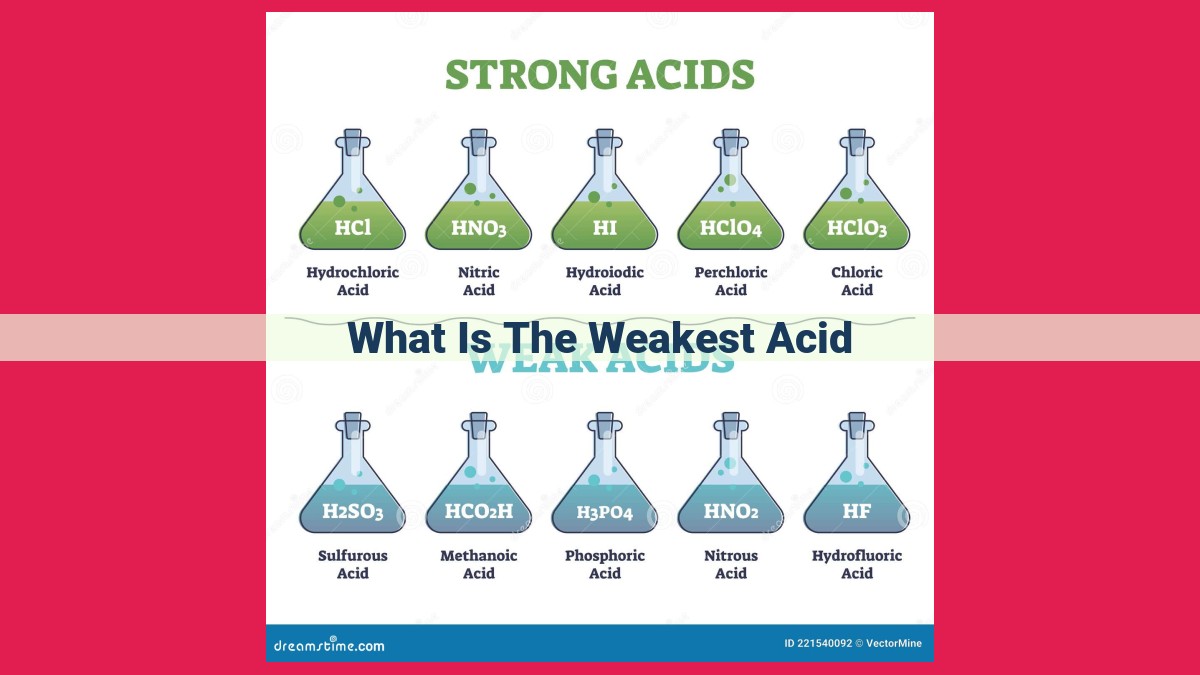
The weakest acid, as defined by its highest pKa value, is the one that dissociates least readily in solution. This means that it holds onto its protons more strongly, resulting in a lower concentration of protons and a weaker acidic effect. The weakest acids, therefore, have conjugate bases that are more stable, making them less likely to react with proton acceptors. Understanding the factors that influence acid strength, such as structure, resonance, and inductive effects, is crucial for identifying the weakest acid in a given scenario.
- Define acids and their role in donating protons
- State the purpose of the article: exploring acid strength and identifying the weakest acid
Best Outline for Blog Post on the Weakest Acid
Acids play a crucial role in our world, donating protons that are essential for countless chemical reactions. Understanding the strength of acids is pivotal, as it governs their reactivity and behavior. In this blog post, we embark on an exploration to identify the weakest acid among all acidic compounds.
Acid Strength
The strength of an acid is a measure of its ability to release protons. Stronger acids dissociate completely, releasing a higher concentration of protons. This strength is quantified by the acid dissociation constant (Ka), a numerical value that reflects the equilibrium between the acid and its conjugate base.
Weakest Acid
The weakest acid is the one with the highest pKa value. pKa is simply the negative logarithm of Ka, and a higher pKa indicates a weaker acid. This means that the weakest acid dissociates less, releasing a lower concentration of protons.
Conjugate Base
When an acid dissociates, it forms a conjugate base. The strength of an acid is inversely related to the strength of its conjugate base. A stronger acid has a weaker conjugate base, and vice versa. This relationship is crucial for understanding the behavior of acids in various chemical systems.
Acid Strength: Understanding the Essence of Acids
Acids, the backbone of chemistry, play a ubiquitous role in numerous biological and industrial processes. Their ability to donate protons, essentially hydrogen ions (H+), is the cornerstone of their significance. So, how do we measure their strength in this proton-donating game? Enter the concept of acid strength, a crucial factor in determining the behavior and effectiveness of acids.
Ka and pKa: The Measuring Sticks of Acid Strength
The strength of an acid is quantified by two key parameters: the acid dissociation constant (Ka) and its negative logarithm, pKa. Ka is an equilibrium constant that reflects the extent to which an acid dissociates in water. In other words, it tells us how much of the acid has released its proton into the solution.
The pKa Puzzle: A Number Game
The pKa value, on the other hand, is a logarithmic measure of acid strength. The lower the pKa, the stronger the acid. This inverse relationship between pKa and acid strength is crucial. Acids with smaller pKa values tend to dissociate more readily, releasing more protons and, thus, exhibiting greater acidity.
The Stronger, the Weaker: A Surprising Twist
Understanding acid strength is not just about knowing which acid is the strongest. It also involves recognizing that the weakest acid is the one with the highest pKa. This might seem counterintuitive at first, but it all boils down to the concept of conjugate base stability.
Weakest Acid: Understanding the Concept and its Significance
Acids, known for their ability to donate protons, play a crucial role in various chemical reactions. But not all acids are created equal; some are stronger than others. In this blog, we’ll delve into the concept of acid strength and identify the weakest acid.
Understanding Acid Strength
Acid strength is determined by its ability to release protons (H+ ions). Acids that dissociate more readily, releasing higher concentrations of protons, are considered stronger. Two important measures of acid strength are the acid dissociation constant (Ka) and pKa.
Ka represents the equilibrium constant for acid dissociation, indicating the extent to which an acid dissociates in water. pKa is the negative logarithm of Ka and is a convenient way to express acid strength. A lower pKa value indicates a stronger acid, while a higher pKa value signifies a weaker acid.
Defining the Weakest Acid
The weakest acid is the one that has the highest pKa value. This means that it dissociates the least in water, releasing the lowest concentration of protons. The conjugate base of a weak acid is conversely the most stable, meaning it has a strong tendency to hold onto the proton that was donated.
The relationship between pKa and conjugate base stability is inversely proportional. A higher pKa indicates a weaker acid and a more stable conjugate base.
The Importance of Understanding Weakest Acids
Identifying the weakest acid in a particular context is essential for understanding the chemistry of the system. It allows us to predict the extent of acid-base reactions, determine the pH of solutions, and control the reactivity of other chemical species.
In summary, the weakest acid is the one with the highest pKa value. Understanding acid strength, Ka, and pKa is crucial for identifying the weakest acid in a specific chemical scenario. This knowledge empowers us to manipulate and control chemical reactions effectively.
Conjugate Bases: The Silent Partners of Weak Acids
In the world of acids, they have a hidden sidekick called conjugate bases. Let’s meet these silent partners and explore their hidden influence on acid strength.
Imagine you have an acid, like a grumpy proton donating machine. When it donates its proton, it leaves behind a conjugate base, a negative ion that’s ready to soak up that proton like a thirsty sponge.
The inverse relationship between acid strength and conjugate base strength is the key here. The stronger the acid, the weaker its conjugate base. This is because a strong acid readily gives up its protons, leaving behind a weak conjugate base that’s eager to regain them.
So, for the weakest acid, we’re looking for the acid with the strongest conjugate base. Why? Because a weak acid doesn’t readily give up its protons, so its conjugate base must be strong enough to pull them back in.
That’s the beauty of conjugate bases: they balance the act. A strong acid has a weak conjugate base, keeping the proton-donating game in check. A weak acid has a strong conjugate base, ensuring proton exchanges don’t get out of hand.
Acid Dissociation Constant (Ka)
Ka is a crucial parameter in understanding acid strength. It represents the equilibrium constant for the dissociation of an acid in water. The value of Ka indicates the extent to which an acid dissociates into protons (H+) and its conjugate base.
When an acid dissolves in water, it undergoes a dissociation reaction. During this reaction, the acid donates a proton to a water molecule, forming a hydronium ion (H3O+) and a conjugate base. The equilibrium constant for this dissociation reaction is known as the acid dissociation constant, or Ka.
A higher Ka value indicates that the acid dissociates more readily, releasing more protons into the solution. This means that the acid is stronger. Conversely, a lower Ka value corresponds to a weaker acid, which dissociates less and releases fewer protons.
The Ka value provides valuable information about the concentration of protons and conjugate base at equilibrium. The higher the Ka, the higher the concentration of protons and conjugate base in solution. This is because a stronger acid will dissociate more completely, resulting in a higher concentration of its dissociation products.
pKa: Quantifying Acid Strength
Imagine you’re at a party filled with acids and bases, each with their unique personalities and tendencies. Some are eager to donate protons, like bold and extroverted acids, while others are shy and reserved, holding their protons close. The pKa value is like a social status symbol for these acids, indicating how willing they are to part with their protons.
Defining pKa
pKa, short for negative logarithm of the acid dissociation constant, is a numerical expression that quantifies acid strength. It’s simply the negative log of Ka, the equilibrium constant that measures the extent to which an acid dissociates in solution. A lower pKa corresponds to a stronger acid, which dissociates more readily, releasing more protons into the solution. Conversely, a higher pKa signifies a weaker acid, which has a lower tendency to dissociate.
Convenience of pKa
The beauty of pKa lies in its simplicity and convenience. Instead of memorizing bulky Ka values, we can use pKa to instantly compare the strength of different acids. It’s like having a handy yardstick that allows us to quickly rank acids from weakest to strongest. This makes it immensely useful for chemists and other scientists who work with acids on a regular basis.
Stronger and Weaker Acids: Unveiling the Acid Strength Spectrum
In the realm of chemistry, acids play a crucial role as proton donors, releasing these tiny particles into the solution. But not all acids are created equal. Some acids reign supreme with their exceptional proton-donating abilities, while others exhibit a more subdued nature.
The strength of an acid is measured by its acid dissociation constant (Ka), a numerical value that quantifies the acid’s tendency to dissociate in water. The lower the Ka value, the stronger the acid. Conversely, acids with higher Ka values are considered weaker.
Stronger acids have a greater affinity for donating protons, resulting in a higher concentration of free protons or H+ ions in the solution. These acids possess a lower pKa value, which is the negative logarithm of Ka. A lower pKa signifies a stronger acid.
On the other end of the spectrum, weaker acids have a weaker grip on their protons, leading to a lower concentration of free protons in the solution. Their Ka values are higher, and consequently, their pKa values are also higher. Weaker acids are less inclined to donate protons, making them less corrosive and reactive.
The interplay between acid strength and conjugate base stability is a key factor to consider. A conjugate base is the species formed when an acid donates a proton. Stronger acids tend to have weaker conjugate bases, while weaker acids exhibit more stable conjugate bases. This relationship arises from the delicate balance of electron distribution within the molecule.
Understanding the concept of stronger and weaker acids is essential for grasping a variety of chemical phenomena. It allows scientists and researchers to predict the behavior of acids in different reactions and to tailor their applications accordingly.
How Structure, Resonance, and Inductive Effects Power Acid Strength
Acids, the powerhouses of proton donation, vary in their strength, with some being more generous than others. The structure of an acid plays a crucial role in determining its willingness to part with these protons. Let’s dive into the molecular realm to explore how these factors shape acid strength.
Resonance: This magical dance of electrons can stabilize the conjugate base, the species formed when an acid donates a proton. When resonance delocalizes the negative charge over multiple atoms, it weakens the acid by making it less likely to release protons.
Inductive Effects: These invisible forces arise from the tug-of-war between electronegative atoms in a molecule. If an electronegative atom is close to the acidic proton, it can pull electron density away from the proton, making it more difficult to donate and thus strengthening the acid.
The interplay of resonance and inductive effects creates a delicate balance that determines the relative strengths of acids. By understanding these factors, we can predict and manipulate acid behavior, unlocking their potential in various applications.