Hydrolysis Vs. Dehydration Synthesis: Essential Chemical Reactions For Life
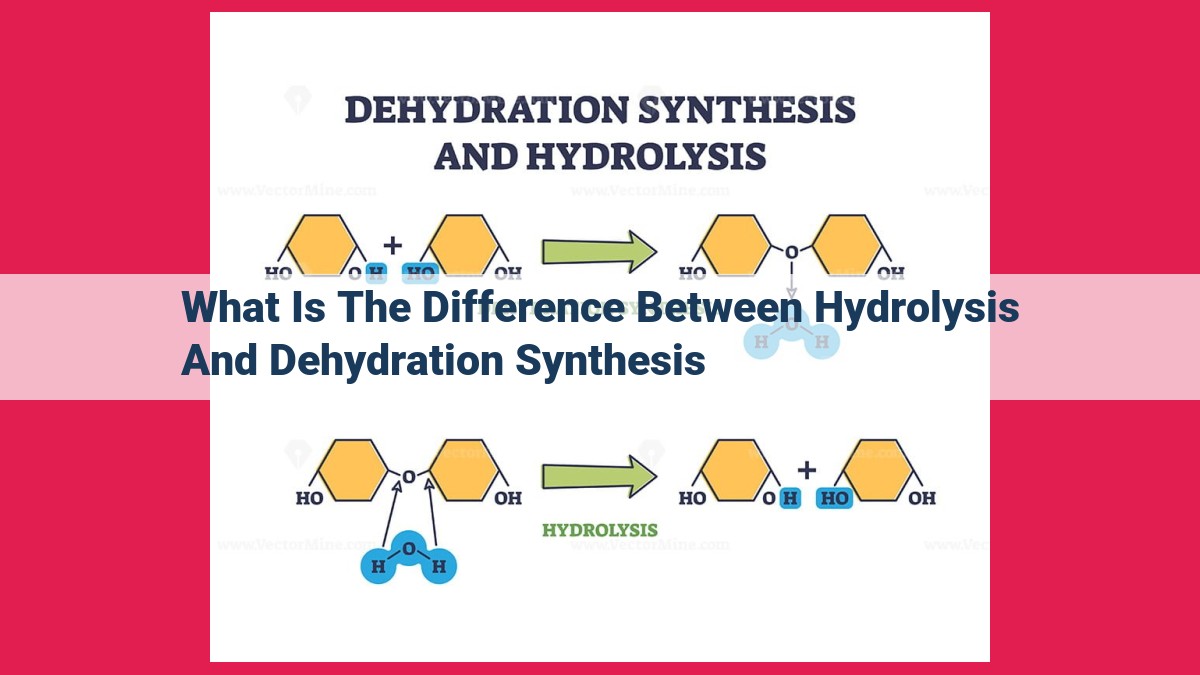
Hydrolysis and dehydration synthesis are contrasting chemical reactions essential in biological systems. Hydrolysis breaks down complex molecules, aiding digestion, metabolism, and catabolism, while dehydration synthesis builds complex molecules, facilitating protein and polysaccharide synthesis, and anabolism. Key differences include their purpose (breakdown vs. synthesis), water involvement (addition vs. removal), and biological roles (breakdown vs. growth). These reactions play a crucial role in regulating biological processes and maintaining cellular homeostasis.
Hydrolysis and Dehydration Synthesis: The Dynamic Duo of Biological Chemistry
In the intricate realm of biological systems, two chemical reactions play pivotal roles in shaping the very essence of life: hydrolysis and dehydration synthesis. These intricate dance partners work hand in hand, orchestrating the breakdown and creation of complex molecules, ultimately driving countless biological processes. Let’s embark on a storytelling journey to unravel the fascinating world of these chemical reactions and their profound impact on life.
Hydrolysis: Breaking Down Barriers
Imagine a hearty meal waiting to be savored. As you take a bite, your body initiates hydrolysis, a process that deconstructs complex molecules with the help of water. This magical potion, known as the “universal solvent,” infiltrates the molecular structure, breaking down the hefty carbohydrates, proteins, and fats into smaller, absorbable units. Hydrolysis is a ubiquitous player in digestion, a symphony of enzymes working diligently to transform food into nutrients that sustain your body.
Beyond digestion, hydrolysis plays a vital role in metabolism, the body’s intricate network of chemical reactions. It’s the scissor that snips apart carbohydrates into glucose, the primary fuel for cells. And in the destructive dance of catabolism, hydrolysis dismantles complex molecules, releasing energy that powers your every move.
Dehydration Synthesis: Building Blocks of Life
Now, let’s flip the script to dehydration synthesis, the master architect that crafts complex molecules by removing water. In this reverse dance, smaller molecules lock hands, shedding water molecules to form bonds that give rise to more complex structures. Dehydration synthesis is the backbone of protein synthesis, the intricate process that assembles amino acids into the proteins that govern every aspect of your body’s function.
Polysaccharides, the storage units for energy, are also sculpted through dehydration synthesis. These complex carbohydrates provide sustained nourishment, fueling your body for prolonged activities. And in growth and repair, dehydration synthesis plays a vital role, weaving together the building blocks of new cells and tissues.
A Tale of Contrasts
Hydrolysis and dehydration synthesis stand as perfect foils, their contrasting functions shaping the biological tapestry. Hydrolysis breaks down, dismantling complex molecules into simpler ones, while dehydration synthesis builds up, synthesizing intricate structures from smaller units. They represent the yin and yang of biological chemistry, the two sides of the molecular coin that maintain the delicate balance of life.
Hydrolysis: Breaking Down Complex Molecules for Biological Processes
In the realm of life, chemical reactions play a pivotal role in orchestrating the symphony of biological processes. Among them, hydrolysis stands out as a crucial reaction responsible for the breakdown of complex molecules, fueling the machinery of life.
Hydrolysis, a word derived from Greek roots meaning “to break down with water,” aptly describes the essence of this reaction. It involves the cleavage of a bond in a complex molecule with the assistance of water molecules. This process results in the splitting of the molecule into two or more smaller components.
The significance of hydrolysis in biological systems cannot be overstated. It serves as the foundation for digestion, the intricate process by which nutrients are broken down into simpler forms that can be absorbed by the body. In the digestive system, hydrolysis reactions catalyzed by enzymes dismantle carbohydrates, proteins, and fats into their building blocks, glucose, amino acids, and fatty acids, respectively.
Beyond digestion, hydrolysis also plays a vital role in metabolism. It unlocks the chemical energy stored within complex molecules, making it available for the body’s energy needs. During metabolism, hydrolysis reactions break down glucose, the primary energy source for cells, into smaller molecules that can be further oxidized to release energy.
Furthermore, hydrolysis is essential for catabolism, the process of breaking down complex molecules into simpler compounds for energy production or waste elimination. Through a series of hydrolysis reactions, cells systematically disassemble macromolecules such as proteins and carbohydrates, liberating their components to be reused or excreted.
In essence, hydrolysis stands as an indispensable chemical reaction in biological systems. It drives digestion, metabolism, and catabolism, providing the building blocks and energy necessary for life to thrive. Its intricate role in these fundamental processes highlights the profound importance of hydrolysis in the tapestry of life.
Dehydration Synthesis: The Mastermind Behind Anabolic Magic
Definition:
Dehydration synthesis, a captivating chemical dance, brings together smaller molecules to forge complex giants. This remarkable process involves a harmonious tango, where water is whisked away as two molecules gracefully intertwine. From the ashes of this molecular waltz, intricate structures emerge, paving the way for life’s intricate tapestry.
The Significance in Biological Systems
Dehydration synthesis plays a pivotal role in the symphony of life, orchestrating the creation of essential molecules that sustain and propel biological systems.
Protein Synthesis:
Proteins, the workhorses of the cellular realm, are masterfully crafted through dehydration synthesis. Amino acids, like tiny building blocks, are fused together, their bonds forged in a molecular ballet. This intricate assembly gives rise to the vast array of proteins that govern countless biological processes, from enzyme catalysis to muscle contraction.
Polysaccharide Synthesis:
Polysaccharides, the energy storehouses of cells, also owe their existence to dehydration synthesis. Sugars, like exquisite jewels, are linked together, forming intricate chains that serve as reservoirs of energy for cellular activities.
Anabolism:
Dehydration synthesis is the driving force behind anabolism, the constructive phase of metabolism. It weaves together simple building blocks into complex molecules, laying the foundation for growth, repair, and reproduction. From the synthesis of DNA to the formation of cell membranes, dehydration synthesis breathes life into the intricate tapestry of biological systems.
Unveiling the Dance of Hydrolysis and Dehydration Synthesis: Opposite Reactions, United in Life
At the heart of biological processes, two chemical reactions stand as polar opposites, yet their harmonious interplay sustains the very fabric of life. Hydrolysis and Dehydration Synthesis are the masterminds behind the breakdown and creation of molecules, orchestrating a delicate balance that underpins every living organism.
Hydrolysis: The Maestro of Molecular Deconstruction
Imagine a complex molecule, a towering skyscraper of atoms, awaiting its hydrolytic demise. In this reaction, water molecules swoop in as wrecking balls, breaking down the bonds that hold the edifice together. Piece by piece, the skyscraper crumbles into its smaller building blocks.
This process plays a pivotal role in our bodies. In digestion, hydrolytic enzymes wield their power to dismantle food molecules into nutrients our cells can absorb. In metabolism, hydrolysis liberates energy from bonds within molecules, fueling our every action. And in catabolism, the final breakdown of biomolecules, hydrolysis prepares them for excretion.
Dehydration Synthesis: The Architect of Molecular Complexity
In stark contrast to hydrolysis, dehydration synthesis embarks on a mission of molecular creation. Picture a scattered pile of building blocks, destined to become a magnificent castle. Dehydration synthesis serves as the architect, removing water molecules to form new bonds between the blocks.
Inside our cells, this reaction is essential for the construction of proteins, the workhorses of biological machinery. It also plays a crucial role in the synthesis of polysaccharides, the energy-storing powerhouses of cells. And just as hydrolysis fuels catabolism, dehydration synthesis drives anabolism, the process of building up complex molecules.
A Tale of Contrasts and Complementarity
Hydrolysis and dehydration synthesis stand as polar opposites, reflecting the delicate balance inherent in biological systems. Hydrolysis tears down molecular structures, while dehydration synthesis builds them up. Water is a crucial player in hydrolysis, while its absence is essential for dehydration synthesis. And ultimately, these reactions serve opposing biological functions: hydrolysis focuses on breakdown for energy or recycling, while dehydration synthesis focuses on growth and repair.
Together, hydrolysis and dehydration synthesis form an intricate dance, shaping the molecular landscape of life. They are the yin and yang of metabolism, the breakdown and buildup that sustain the delicate equilibrium of living organisms.