Understanding The Differences Between Hydrogen Bonds And Covalent Bonds: A Detailed Comparison
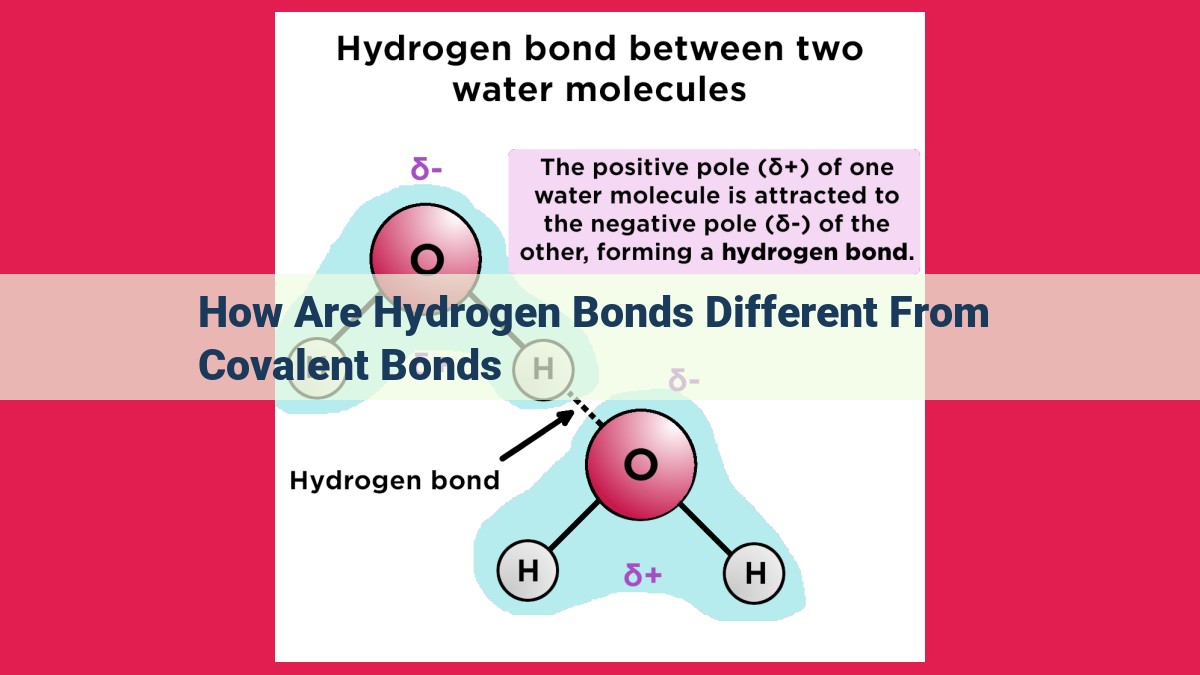
Hydrogen bonds are distinct from covalent bonds due to stark differences in bond strength, distance, geometry, polarity, lifetime, bonding limits, and directionality. Covalent bonds are stronger, shorter, exhibit well-defined bond angles, and have longer lifetimes. Hydrogen bonds are weaker, longer, have less defined bond angles, are polar, have shorter lifetimes, have a limit of two bonds per atom, and exhibit more variable directionality.
Bond Strength: A Tale of Two Bonds
In the vast realm of chemistry, bonds are the invisible chains that hold atoms together, forming the building blocks of our world. Among these bonds, two stand out: covalent bonds and hydrogen bonds. While both are essential to life, they possess starkly different strengths, giving rise to a captivating tale of two bonds.
Covalent bonds, forged by the sharing of electrons between atoms, are the powerhouses of the chemical world. Their bond energies, typically ranging from 200 to 1000 kilojoules per mole (kJ/mol), reflect their immense strength. This strength is evident in the stability of molecules such as diamond, the hardest known natural substance, and water, the lifeblood of our planet.
In contrast, hydrogen bonds are more akin to ethereal whispers. Formed by the electrostatic attraction between a hydrogen atom and an electronegative atom like oxygen or nitrogen, hydrogen bonds possess bond energies in the range of 20-30 kJ/mol. This makes them considerably weaker than covalent bonds. Yet, despite their delicate nature, hydrogen bonds play a crucial role in shaping our world, from stabilizing the structure of DNA to driving the interactions between water molecules.
Bridging the Gap: Distance and Geometry
In the realm of molecular architecture, the dance of atoms is defined by the bonds they form. Among these bonds, covalent bonds and hydrogen bonds stand out, each with its unique characteristics. While both play crucial roles in shaping the world around us, their distance and geometry reveal a tale of contrasts.
Covalent Bonds: The Intimate Embrace
Covalent bonds arise from the intimate embrace of atoms, when they share electrons to form a cohesive unit. These bonds are characterized by their short distances, typically less than 0.2 nanometers. This coziness is a result of the strong attraction between the shared electrons and the positively charged nuclei.
Hydrogen Bonds: A Distant Dance
In contrast to the intimate embrace of covalent bonds, hydrogen bonds are like a gentle waltz, where atoms maintain a respectful distance from each other. These bonds form when a hydrogen atom, sandwiched between two electronegative atoms, acts as a bridge. The distance between the hydrogen atom and the electronegative atoms is significantly longer than in covalent bonds, often exceeding 0.25 nanometers.
Covalent Radii: The Molecular Ruler
The covalent radius of an atom, a measure of its outer electron shell, plays a pivotal role in determining bond length. Atoms with larger covalent radii have longer bonds due to the increased distance between their nuclei. This concept helps explain the variations in bond lengths observed in different molecules.
Variable Geometry: Hydrogen Bonds Adapt
Unlike the well-defined angles of covalent bonds, hydrogen bonds exhibit a more flexible geometry. The arrangement of surrounding molecules influences the direction and strength of these bonds. This adaptability allows hydrogen bonds to form in a wide range of molecular environments, contributing to the complexity and diversity of life.
The Structural Dance: Bond Angles and Geometries
Covalent Bonds: A Precise Pas de Deux
Covalent bonds, forged by the intertwined embrace of electron pairs, exhibit well-defined bond angles. This precision stems from the orientation of the atomic orbitals that contribute to the bond. Like dancers following a choreographed routine, the orbitals align perfectly, dictating the angle at which the atoms bond.
Hydrogen Bonds: A Flexible Foxtrot
In contrast to the rigid structure of covalent bonds, hydrogen bonds engage in a more fluid dance. These bonds form between a partially positive hydrogen atom and a highly electronegative atom, such as oxygen or nitrogen. The geometry of these bonds is not as rigidly dictated as in covalent bonds, as it depends on the arrangement of the surrounding molecules.
Visualizing the Dance
To illustrate the contrasting angles and geometries, imagine two dancers performing a covalent bond. Their bodies are precisely aligned, creating a sharp and well-defined angle. Now, envision two dancers performing a hydrogen bond. Their movements are more fluid, adjusting to the presence of other dancers and the surrounding environment, creating a less specific geometry.
Consequences of the Dance
The distinct bond angles and geometries of covalent and hydrogen bonds have significant implications for the structures of molecules and materials. Covalent bonds, with their precise angles, determine the overall shape of molecules, while hydrogen bonds, with their more flexible geometries, influence the interactions between molecules. These differences contribute to the vast diversity of structures found in nature.
Polarity: A Tale of Charges
In the realm of chemical bonds, the concept of polarity plays a crucial role in understanding their behavior. Bonds can be either polar or nonpolar, depending on the distribution of electrons between the atoms involved.
Covalent Bonds: A Spectrum of Polarities
Covalent bonds arise when atoms share electron pairs. The polarity of a covalent bond depends on the difference in electronegativity between the atoms – the ability of an atom to attract electrons towards itself. If the electronegativity difference is zero, the bond is nonpolar, with the electrons evenly shared. However, if the difference is not zero, the bond is polar, with the electrons shifted towards the more electronegative atom.
Hydrogen Bonds: A Polar Interplay
Hydrogen bonds are a special type of dipole-dipole interaction that occurs between a hydrogen atom covalently bonded to an electronegative atom (such as oxygen or nitrogen) and another electronegative atom. The electronegative atom pulls electrons away from the hydrogen, creating a partial positive charge on the hydrogen and a partial negative charge on the electronegative atom. This polarity enables hydrogen bonds to form between molecules.
Contrasting Polarities: Covalent vs. Hydrogen Bonds
The polarity of covalent and hydrogen bonds differs significantly. Covalent bonds can have varying degrees of polarity, depending on the electronegativity difference between the atoms. Hydrogen bonds, on the other hand, are inherently polar, with a fixed dipole moment. This difference in polarity has a profound impact on the properties of molecules and their interactions.
The Temporal Dance: Bond Lifetime
In the world of molecular bonds, time plays a crucial role in determining their existence and behavior. Covalent bonds, the sturdy pillars of molecular structures, stand tall for milliseconds to years, anchoring atoms together with unwavering strength. In contrast, hydrogen bonds, the ephemeral connectors, dance a fleeting existence, lasting mere picoseconds to nanoseconds.
This stark difference in bond lifetime stems from the nature and strength of these bonds. Covalent bonds, forged by the sharing of electrons between atoms, form stable connections with well-defined bond lengths and angles. Their bond strength resists any attempt at separation, allowing molecules to maintain their integrity over extended periods.
Hydrogen bonds, on the other hand, arise from a more subtle electrostatic attraction between a hydrogen atom and an electronegative atom. Their weaker bond strength allows for their formation and breaking on a much faster timescale. This dynamic nature enables hydrogen bonds to facilitate essential processes in biological systems, such as the folding of proteins and the formation of DNA double helices.
Various factors can influence bond lifetime. Bond strength is a major determinant, with stronger bonds typically having longer lifetimes. Environmental conditions also play a role, as temperature and solvent polarity can affect the stability of bonds.
Understanding bond lifetime is essential for comprehending the behavior and properties of molecules. It helps us unravel the secrets of chemical reactions, molecular recognition, and the intricate dance of life at the molecular level.
Bonding Limits: Counting Connections in the Molecular World
In the vast tapestry of chemical interactions, atoms dance together, forming myriad molecular bonds. While covalent bonds reign supreme, capable of forging multiple connections, their hydrogen bond counterparts face a strict bonding limit: a maximum of two bonds per atom. Understanding these limitations unveils crucial insights into the structure and behavior of molecules.
Covalent Bonds: A Multiplicity of Connections
Picture a child playing with building blocks, connecting them in a myriad of ways. Similarly, atoms form covalent bonds by interlocking their shared electron pairs. This flexibility allows multiple covalent bonds to emerge, creating molecules with complex architectures. Carbon, for instance, can boast four covalent bonds, enabling it to form the backbone of organic molecules.
Hydrogen Bonds: A Tale of Two Limbs
Unlike covalent bonds, hydrogen bonds arise from a partial positive charge on a hydrogen atom interacting with a partially negative region elsewhere in the molecule. This electrostatic attraction is far weaker than a covalent bond, limiting each hydrogen atom to forming a maximum of two hydrogen bonds.
Implications for Molecular Structure
These bonding limitations profoundly impact molecular structure. The versatility of covalent bonds allows atoms to connect in diverse ways, giving rise to a wide range of molecular shapes and functionalities. In contrast, the constrained hydrogen bonding influences molecular arrangements, often leading to specific orientations and interactions. For example, the double hydrogen bonding capacity of water molecules enables them to form intricate networks, shaping its unique properties.
Delving into the contrasting bonding limits of covalent and hydrogen bonds provides a glimpse into the intricate web of chemical interactions that underpin the molecular world. By appreciating the multiplicity of covalent bonds and the duality of hydrogen bonding, we gain a deeper understanding of the structures, properties, and behaviors of myriad substances, from water to DNA.
Directionality: A Matter of Choice
When it comes to bonding directionality, covalent bonds and hydrogen bonds take different paths. Covalent bonds are formed when atoms share electrons in specific directions, guided by the hybridization of their orbitals. This hybridization creates specific bond angles and geometries, as you’ll see in the upcoming section.
Hydrogen bonds, on the other hand, are more flexible in their directionality. They form between an electronegative atom (such as oxygen or nitrogen) and a hydrogen atom bonded to another electronegative atom. The direction of the hydrogen bond is influenced by the surrounding molecules and the availability of hydrogen bond acceptors.
To illustrate this difference, imagine a dance floor. Covalent bonds are like couples who follow a specific dance pattern, moving in a synchronized fashion. Hydrogen bonds, on the other hand, are like solo dancers who can change their steps and directions based on the music and the position of other dancers on the floor.