Mastering Molecule To Mole Conversion: A Comprehensive Guide
To convert molecules to moles, we use Avogadro’s number (6.022 x 10^23 entities per mole) as the conversion factor. The formula is: moles = molecules / Avogadro’s number. Conversely, to convert moles to molecules, we multiply moles by Avogadro’s number. Understanding molar mass and molecular weight is crucial. Molar mass is the mass of one mole of a substance, while molecular weight is the mass of one molecule. By dividing the mass of the substance by its molar mass, we can convert grams to moles. Conversely, multiplying moles by the molar mass gives us grams. These conversions are essential for stoichiometric calculations, which involve determining the precise amounts of reactants and products in chemical reactions.
Molecule-to-Mole Conversions: The Foundation of Chemistry
In the vast symphony of chemistry, precise molecule-to-mole conversions play the role of meticulous conductors. They allow us to navigate the intricate dance of chemical reactions, understand the language of stoichiometry, and unlock the secrets hidden within substances.
Imagine a kitchen. An enticing recipe calls for 3 cups of flour. But what if we only have it in grams? We reach for a conversion factor, a bridge that translates between different units, allowing us to accurately measure the flour we need. Similarly, in chemistry, Avogadro’s number serves as the quintessential conversion factor, enabling us to translate between molecules and moles, the SI unit for the amount of substance.
Stoichiometry, the language of chemical reactions, demands precise understanding of molecule-to-mole conversions. It unravels the dance of elements, revealing the exact proportions in which they combine and react. Without these conversions, we’d be left stumbling through chemical reactions, unable to harness their transformative power.
Avogadro’s Number: The Gateway to Understanding the Microscopic World
In the realm of chemistry, the concept of molecules and moles is crucial in unraveling the intricate interactions that govern the universe. Avogadro’s number, a fundamental constant, acts as a bridge between these two seemingly disparate realms, enabling us to navigate the vastness of the microscopic world.
Defined as the number of atoms or molecules present in exactly 12 grams of carbon-12, Avogadro’s number, denoted by Nₐ, is a staggering 6.022 x 10^23. This colossal figure represents the unfathomable number of particles that constitute even the tiniest amounts of matter.
The Mole: A Unit of Measure for the Realm of Substances
Based on the groundbreaking discovery of Avogadro’s number, scientists introduced the mole as the SI unit for measuring the amount of substance. A mole, symbolized by mol, is defined as the quantity of a substance that contains Nₐ specified entities, whether atoms, molecules, ions, or electrons.
This concept provides a universal standard for quantifying the abundance of substances, providing a common language for chemists to communicate and compare their findings. The mole serves as a bridge between the macroscopic and microscopic scales, allowing us to relate the observable mass and volume of substances to the underlying particles that compose them.
Molar Mass and Molecular Weight: Uncovering the Essence of Chemical Substances
In the enchanting realm of chemistry, where atoms dance and molecules intertwine, understanding the relationship between molar mass and molecular weight is paramount to unlocking the secrets of chemical substances. These concepts, like twinkling stars in the celestial tapestry of chemistry, guide us through the intricate maze of chemical equations and quantitative analysis.
Defining Molar Mass
Molar mass, often referred to as the weight of one mole, represents the mass of one mole of a substance. It is expressed in grams per mole (g/mol). Picture a crowd of identical molecules, each carrying an infinitesimal mass. The molar mass is the collective mass of all these molecules in a single mole.
Molecular Weight Revisited
Molecular weight, on the other hand, is the sum of the atomic weights of all the atoms that make up a molecule. It is expressed in atomic mass units (amu). Imagine a weighing scale, where each atom’s weight contributes to the overall molecular weight.
The Connection Unraveled
The molar mass and molecular weight of a substance are closely intertwined. For molecular compounds, the molar mass is numerically equal to the molecular weight. This harmonious relationship arises because one mole of a molecular compound contains Avogadro’s number (6.022 x 10^23) molecules, and each molecule has the same molecular weight.
For ionic compounds, however, the molar mass and molecular weight are not numerically equal. Ionic compounds consist of ions, which are electrically charged particles. The molar mass of an ionic compound includes the masses of all the ions in its formula unit, while the molecular weight refers to the neutral molecule that forms when the ions combine.
Significance in Chemistry
Understanding molar mass and molecular weight is essential for various reasons:
- Stoichiometry Calculations: These calculations involve determining the quantitative relationships between reactants and products in chemical reactions. Molar mass allows us to convert between the number of moles and mass of substances, ensuring precise calculations.
- Solution Concentration: Molarity, a measure of solution concentration, is expressed as moles of solute per liter of solution. Knowing the molar mass enables us to prepare solutions with specific concentrations.
- Empirical Formula Determination: Empirical formulas represent the simplest whole-number ratio of elements in a compound. By comparing the molar mass of a compound with its molecular weight, we can derive its empirical formula.
Conversions between Molecules and Moles
In the realm of chemistry, understanding the relationship between molecules and moles is paramount for precise calculations. Molecules represent the basic building blocks of substances, while moles define the quantity of those molecules. interconverting between these units is crucial for stoichiometric calculations, which dictate the proper proportions of reactants and products in chemical reactions.
Molecule-to-Mole Conversion
Formula: Number of molecules ÷ Avogadro’s number (6.022 x 10^23 molecules/mole)
Example: Convert 1.2 x 10^24 molecules of carbon dioxide (CO2) to moles:
1.2 x 10^24 molecules CO2 ÷ 6.022 x 10^23 molecules/mole = _**2 moles CO2**_
Mole-to-Molecule Conversion
Formula: Number of moles × Avogadro’s number
Example: Convert 0.5 moles of glucose (C6H12O6) to molecules:
“`
0.5 moles C6H12O6 × 6.022 x 10^23 molecules/mole = 3.011 x 10^23 molecules C6H12O6
Conversions Involving Mass
Calculating the amount of a substance is crucial for balancing chemical equations and determining the mass of reactants and products. Conversions between mass and moles allow us to bridge the gap between the macroscopic and microscopic scales.
Grams-to-Moles Conversion
To convert grams of a substance to moles, we use the following formula:
Moles = Grams ÷ Molar Mass
The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). It is a unique property for each substance.
Example: Convert 100 grams of sodium chloride (NaCl) to moles.
Molar mass of NaCl = 58.44 g/mol
Moles of NaCl = 100 g ÷ 58.44 g/mol = 1.71 moles
Moles-to-Grams Conversion
To convert moles of a substance to grams, we use the formula:
Grams = Moles × Molar Mass
Example: Convert 0.5 moles of glucose (C₆H₁₂O₆) to grams.
Molar mass of glucose = 180.16 g/mol
Grams of glucose = 0.5 moles × 180.16 g/mol = 90.08 grams
Molecule-to-Mole Conversions: A Guide to Understanding Chemistry
In the realm of chemistry, converting between molecules and moles is a fundamental skill. It’s like translating a language to unlock the secrets hidden within chemical reactions. This guide will help you master the art of molecule-to-mole conversions, making you a proficient chemist.
Avogadro’s Number and the Mole
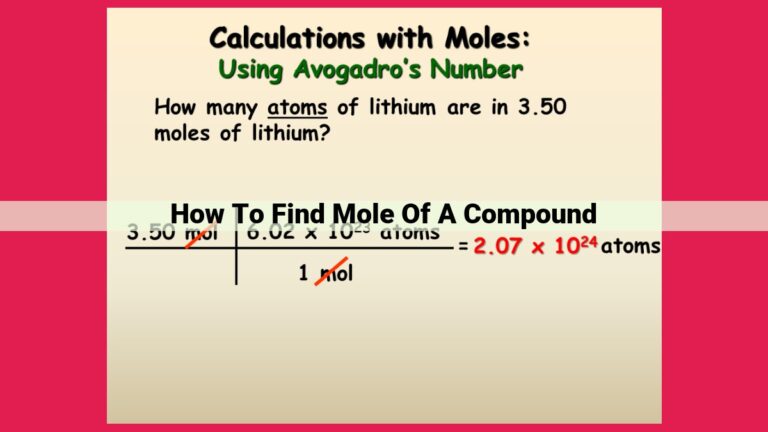
At the heart of molecule-to-mole conversions lies Avogadro’s number. Imagine a colossal collection of 6.022 x 10^23 entities. That’s Avogadro’s number, and it represents the number of entities (atoms, molecules, ions) found in one mole of any substance.
The mole is the SI unit for the amount of substance. It’s the equivalent of a “dozen” for eggs or a “pair” for shoes, but for molecules. One mole of a substance contains exactly 6.022 x 10^23 entities of that substance.
Molar Mass and Molecular Weight
Molar mass is the mass of one mole of a substance, expressed in grams. It’s like the weight of a box containing 6.022 x 10^23 marbles. The molecular weight of a substance is simply the sum of the atomic weights of its constituent atoms.
Conversions between Molecules and Moles
Now, let’s dive into the actual conversions. To convert molecules to moles, simply divide the number of molecules by Avogadro’s number:
Number of moles = Number of molecules / Avogadro's number
For example, if you have 1.2 x 10^24 molecules of water (H2O), you can calculate the number of moles as follows:
Number of moles = 1.2 x 10^24 molecules / 6.022 x 10^23 molecules/mol
= 2 moles
To convert moles to molecules, multiply the number of moles by Avogadro’s number:
Number of molecules = Number of moles x Avogadro's number
For instance, let’s convert 0.5 moles of carbon dioxide (CO2) to molecules:
Number of molecules = 0.5 moles x 6.022 x 10^23 molecules/mol
= 3.011 x 10^23 molecules
Example Calculation
Let’s walk through a complete example. Suppose you want to convert 2.7 x 10^22 molecules of methane (CH4) to moles.
- Convert molecules to moles:
Number of moles = 2.7 x 10^22 molecules / 6.022 x 10^23 molecules/mol
= 0.045 moles
- Check your answer:
To ensure accuracy, let’s convert the 0.045 moles back to molecules:
Number of molecules = 0.045 moles x 6.022 x 10^23 molecules/mol
= 2.7 x 10^22 molecules
Our answer matches the original number of molecules, confirming the accuracy of our conversion.
Mastering molecule-to-mole conversions is crucial in chemistry. By understanding the concepts of Avogadro’s number, molar mass, and the formulas involved, you can perform these conversions confidently. Remember, accurate conversions are the foundation for accurate chemical calculations, unlocking the secrets of the molecular world.