How To Draw A Bohr Model: A Step-By-Step Guide For Understanding Atomic Structure
Drawing a Bohr model involves understanding atomic structure. Start by determining the number of electrons from the atomic number. Arrange electrons in shells and subshells guided by quantum numbers. For example, in helium, the two electrons occupy the first shell (n=1), with one in the s-subshell (l=0) and the other in the p-subshell (l=1). Electron spin is indicated by up or down arrows. Bohr models provide a simplified representation of atomic structure, aiding in understanding the properties and behavior of atoms.
Definition and Importance of Bohr Models:
- Define Bohr models as simplified representations of atomic structure.
- Explain their importance in understanding the properties and behavior of atoms.
Unraveling the Secrets of the Atom: A Journey through Bohr Models
Atoms, the fundamental building blocks of matter, possess a fascinating internal structure. Understanding this structure is crucial in comprehending the properties and behavior of various substances. Bohr models, introduced by physicist Niels Bohr, provide us with a simplified yet powerful representation of atomic architecture.
What is a Bohr Model?
Imagine a miniature solar system within an atom. The nucleus, like the sun, lies at the center, harboring protons (positively charged particles) and neutrons (neutral particles). Surrounding the nucleus, like planets orbiting a star, are electrons, negatively charged particles.
The arrangement of electrons is not random but follows specific rules. Electrons occupy discrete energy levels called shells. Each shell can accommodate a certain number of electrons, with the outermost shell holding the most. The distribution of electrons within these shells is known as the electron configuration.
The Importance of Bohr Models
Bohr models are valuable tools for understanding the properties of atoms and their behavior in chemical reactions. They provide insights into:
- The number of protons and electrons in an atom, determining its atomic number.
- The valence electrons in the outermost shell, which dictate an atom’s chemical reactivity.
- The stability and reactivity of different elements based on the number and arrangement of their electrons.
Drawing a Bohr Model
To draw a Bohr model, calculate the number of electrons based on the atomic number. Arrange them in concentric shells and subshells, guided by quantum numbers that describe their specific properties. Quantum numbers, like addresses for electron orbitals, specify their energy level, shape, and orientation.
Bohr models offer a foundational understanding of atomic structure, facilitating our exploration of the chemical world. They empower us to predict the behavior of atoms, design new materials, and develop innovative technologies that shape our lives.
Delving into the Heart of Atoms: Understanding their Components
At the core of every atom lies a nucleus, a densely packed region where the protons and neutrons reside. Protons, carrying a positive charge, define the atom’s atomic number, which determines its identity on the periodic table. Neutrons, on the other hand, lack an electrical charge and primarily contribute to the atom’s atomic mass.
Orbiting the nucleus are electrons, negatively charged particles arranged in distinct energy levels called shells. Each shell can accommodate a specific number of electrons, forming concentric circles around the nucleus. The distribution of electrons in these shells is known as electron configuration, which plays a crucial role in determining an atom’s chemical properties.
Electrons occupy shells based on their energy levels, with those closest to the nucleus having the lowest energy. The number of electrons in each shell follows the aufbau principle, which states that electrons fill orbitals in order of increasing energy. This layered arrangement creates a stable and predictable structure for atoms.
Understanding the components of an atom provides a foundation for comprehending its behavior and interactions with other atoms. By unraveling the mysteries of the atomic nucleus and its orbiting electrons, we unlock a deeper appreciation for the fundamental building blocks of our universe.
Quantum Numbers: Describing Electron Properties
In the captivating world of atomic structure, quantum numbers emerge as crucial players in unraveling the enigmatic properties of electrons. They provide a numerical address system, assigning unique quantum attributes to each electron within an atom.
Principal Quantum Number (n)
Think of the principal quantum number (n) as a house number, indicating the shell in which an electron resides. Each house corresponds to an energy level, with lower numbers representing shells closer to the nucleus and higher numbers signifying shells farther away.
Angular Momentum Quantum Number (l)
The angular momentum quantum number (l) mimics a dance instructor, defining the shape of the electron’s subshell within each shell. Subshells come in various forms, such as spherical (s), dumb-bell-like (p), or fancy knots (d, f, and so on), each with its unique spatial orientation.
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) is the address within the subshell, specifying the direction of the subshell’s orientation in space. It’s like giving precise coordinates—up, down, left, or right—to locate the electron’s exact whereabouts.
Spin Quantum Number (ms)
Lastly, the spin quantum number (ms) adds a delightful twist to the electron’s identity. It’s like the electron’s inherent character, describing its intrinsic spin. Electrons can either spin clockwise or counterclockwise, denoted by the quantum numbers +1/2 and -1/2, respectively.
Delving into the realm of quantum numbers unveils the intricate dance of electrons within atoms. These enigmatic numbers decode the electron’s address, shape, orientation, and even its inherent spin. Understanding quantum numbers is a passport to comprehending the fundamental nature of atomic structure and the captivating world of quantum mechanics.
Drawing a Bohr Model:
- Explain the steps involved in drawing a Bohr model.
- Instruct on determining the number of electrons based on atomic number.
- Guide the arrangement of electrons in shells and subshells.
- Provide instructions on using quantum numbers to specify electron orientations and spin.
- Describe how to calculate atomic mass from the number of neutrons and protons.
Understanding the Structure of Atoms with Bohr Models
The enigmatic world of atomic structure has intrigued scientists for centuries, leading to the development of various models to explain the behavior and properties of atoms. Among these models, the Bohr model stands out as a pivotal stepping stone in our understanding of the atomic realm.
Components of an Atom: The Building Blocks of Matter
To grasp the concept of a Bohr model, it’s essential to dive into the fundamental components of an atom. The nucleus, the heart of the atom, is where protons and neutrons reside. Protons, with their positive charges, determine the atomic number of an element, while neutrons, devoid of charge, contribute to the atomic mass.
Electrons, on the other hand, are negatively charged particles that dance around the nucleus in specific energy levels known as shells. The arrangement of electrons in these shells, called electron configuration, plays a crucial role in shaping the chemical behavior of an element.
Quantum Numbers: Guiding the Electron Dance
To describe the intricate motion of electrons, scientists employ a set of quantum numbers. The principal quantum number (n) specifies the shell number, while the angular momentum quantum number (l) determines the subshell shape. The magnetic quantum number (ml) provides information about the subshell’s orientation, and the spin quantum number (ms) indicates the electron’s intrinsic spin.
Drawing a Bohr Model: Unveiling the Atomic Landscape
Armed with this knowledge, let’s embark on the fascinating journey of drawing a Bohr model:
-
Determine the Number of Electrons: The atomic number of an element reveals the number of electrons orbiting its nucleus.
-
Arranging Electrons in Shells: Electrons fill shells starting from the innermost (n = 1) and progress outward. Each shell can accommodate a specific number of electrons, with the outermost shell holding the valence electrons responsible for chemical bonding.
-
Subshells and Orientations: Within each shell exist subshells, designated by the value of l. These subshells have specific shapes and energy levels, influencing the electron’s orientation in space.
-
Electron Spin: The spin quantum number (ms) accounts for the electron’s intrinsic angular momentum, which can be either “spin up” or “spin down.”
-
Calculating Atomic Mass: The atomic mass of an element can be estimated by adding the number of protons and neutrons present in its nucleus.
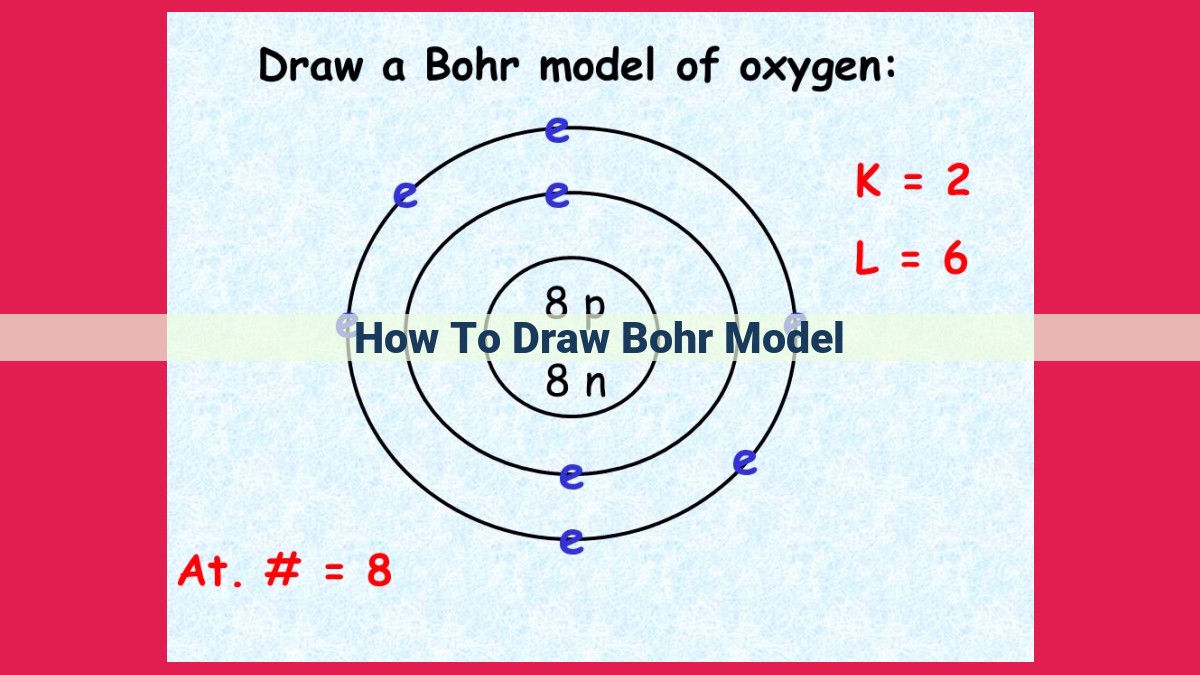
Example: Helium’s Atomic Structure
To illustrate the application of these concepts, let’s construct a Bohr model for helium (He):
-
Helium’s atomic number is 2, indicating it has two protons and two electrons.
-
The two electrons occupy the first shell (n = 1), as it can accommodate a maximum of two electrons.
-
Within the first shell, there is only one subshell (l = 0), which is spherical in shape.
-
The two electrons have opposite spins (ms = +1/2 and -1/2).
-
Helium’s atomic mass is 4, as it contains two protons and two neutrons.
Bohr models provide a simplified yet insightful representation of atomic structure, aiding us in understanding the fundamental properties of elements and their chemical behavior. While more sophisticated models have emerged over time, Bohr models remain invaluable tools for visualizing the intricacies of the atomic realm and laying the foundation for further exploration in atomic physics and chemistry.
Unraveling the Atomic Universe: A Comprehensive Guide to Bohr Models
Definition and Significance
In the realm of atomic structure, Bohr models emerge as simplified representations that illuminate the intricacies of atomic behavior. These models, conceptualized by Niels Bohr, provide a vivid portrayal of atomic components and their interactions. By understanding Bohr models, we delve into the fundamental properties that govern the building blocks of our universe.
Components of an Atom
At the heart of every atom lies its nucleus, a densely packed region containing protons (positively charged) and neutrons (neutral particles). Protons, with their positive charges, dictate the atomic number, which uniquely identifies each element. Neutrons, on the other hand, influence the atom’s atomic mass.
Orbiting the nucleus are electrons, negatively charged particles that arrange themselves in concentric shells around the nucleus. This arrangement, known as electron configuration, is a crucial factor in determining the atom’s chemical properties.
Quantum Numbers: Deciphering Electron Behavior
Quantum numbers play a pivotal role in unraveling the characteristics of electrons. These numbers describe specific properties:
- Principal quantum number (n): Indicates the shell number where the electron resides.
- Angular momentum quantum number (l): Reveals the subshell shape within the shell.
- Magnetic quantum number (ml): Specifies the orientation of the subshell within the shell.
- Spin quantum number (ms): Describes the electron’s spin, either “up” or “down.”
Crafting a Bohr Model: A Step-by-Step Guide
Drawing a Bohr model empowers us to visualize atomic structure. Here’s a step-by-step guide:
- Determine Electron Count: Calculate the number of electrons using the atomic number.
- Arrange Electrons: Populate the shells and subshells based on quantum numbers.
- Specify Electron Orientations: Assign the magnetic quantum number (ml) to indicate subshell orientation.
- Calculate Atomic Mass: Sum the number of protons and neutrons to determine the atomic mass.
Unveiling Helium’s Atomic Structure
Let’s apply our knowledge to draw a Bohr model for helium:
Step 1: Helium has an atomic number of 2, indicating two electrons.
Step 2: The electrons occupy the first shell (n = 1).
Step 3: Both electrons reside in the s subshell (l = 0) within the first shell.
Step 4: Since there is only one s subshell (ml = 0), both electrons align in the same orientation.
Step 5: Helium has 2 protons and 2 neutrons, resulting in an atomic mass of 4.
Bohr models empower us to fathom the intricate world of atomic structure. By understanding their components and the principles governing electron behavior, we gain profound insights into the fundamental building blocks of matter.