Ph Calculation In Buffer Systems: The Henderson-Hasselbalch Equation
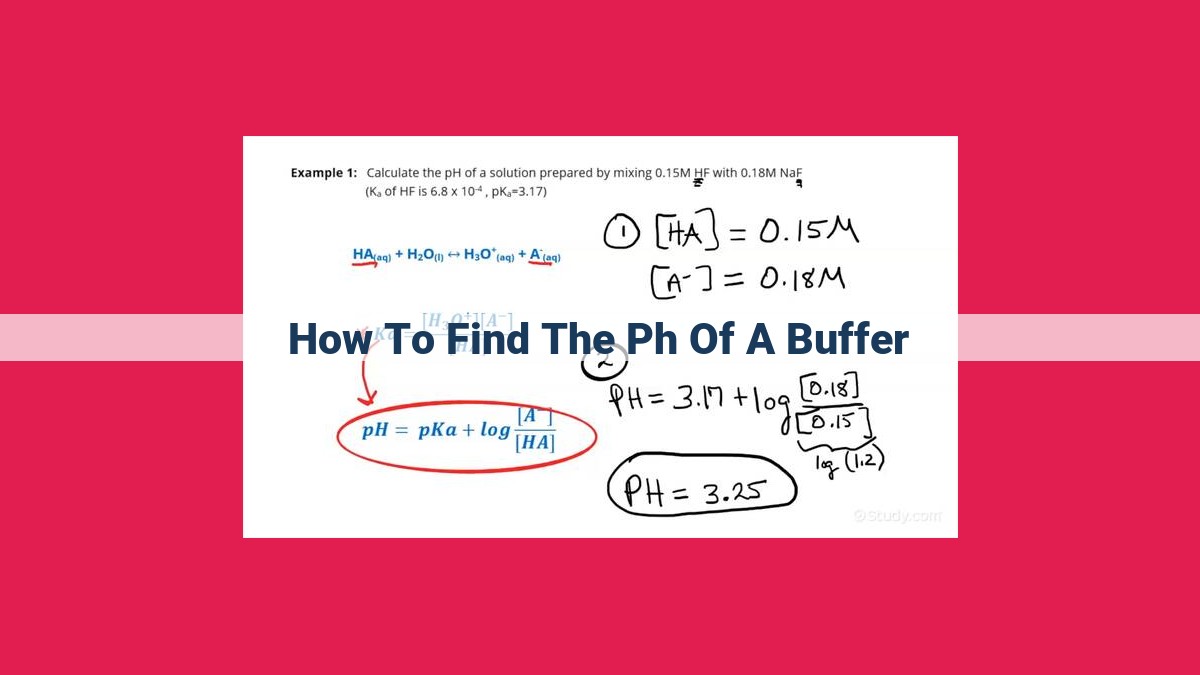
To find the pH of a buffer system, you can use the Henderson-Hasselbalch equation: pH = pKa + log([A-] / [HA]), where pKa is the dissociation constant of the weak acid (HA) in the buffer, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid. By knowing the pKa and the relative concentrations of the buffer components, you can calculate the pH of the buffer.
The Wonders of Buffer Systems: Maintaining the Delicate Balance of pH
Imagine you’re hosting a grand party with a lively mix of guests, each with their unique preferences. Some prefer the sweet taste of lemonade, while others enjoy the tangy kick of vinegar. To ensure everyone has a harmonious experience, you need a “buffer” that can neutralize excess sweetness or acidity, keeping the party’s atmosphere in perfect balance.
In the world of chemistry, buffer systems play a similar role, maintaining the delicate balance of pH in various environments. Buffer systems consist of a weak acid and its conjugate base or a weak base and its conjugate acid. They work their magic by absorbing excess hydrogen ions (H+) or hydroxide ions (OH-), preventing drastic pH changes.
Weak Acid Buffer Systems
Weak acid buffer systems are formed when a weak acid, such as acetic acid, is combined with its conjugate base, acetate. These systems are especially effective in the pH range below the weak acid’s pKa. The pKa is a constant that represents the strength of a weak acid and indicates the pH at which it is 50% ionized.
When the pH is less than the pKa, more of the weak acid is present, while the amount of conjugate base is relatively small. Conversely, when the pH is greater than the pKa, more of the conjugate base is present, and the weak acid is minimal. This dynamic balance ensures that both the weak acid and its conjugate base can neutralize any excess H+ or OH- ions, effectively buffering the pH within a certain range.
Explain how pH influences the relative amounts of acid and conjugate base.
How pH Influences the Relative Amounts of Acid and Conjugate Base
In the realm of chemistry, weak acid buffer systems play a crucial role in maintaining a stable pH, a measure of the acidity or basicity of a solution. These systems consist of a weak acid (HA) and its conjugate base (A-), both present in significant concentrations. They act as a buffer against drastic pH changes when small amounts of strong acids or bases are added.
The pH of a weak acid buffer system is intimately connected to the relative amounts of the acid and its conjugate base. When the pH is low, the concentration of HA is higher, while the concentration of A- is lower. This is because protons (H+) are more likely to be present in a low-pH environment, and they react with A- to form HA.
Conversely, when the pH is high, the concentration of A-_ is higher, while the **concentration of HA is lower. This is because hydroxyl ions (OH-) are more likely to be present in a high-pH environment, and they react with HA to form A- and water.
This dynamic interplay between pH and the relative amounts of acid and conjugate base is essential for maintaining a stable pH. When a small amount of strong acid is added to the buffer system, the increased H+ concentration shifts the equilibrium towards HA formation, thus counteracting the addition of H+. Similarly, when a small amount of strong base is added, the increased OH- concentration shifts the equilibrium towards A- formation, resisting the addition of OH-.
Understanding this fundamental relationship between pH and the relative amounts of acid and conjugate base is key to appreciating the role of weak acid buffer systems in maintaining a predictable and stable pH in various chemical and biological processes.
Buffer Systems: Maintaining the Delicate Balance of pH
In our complex world, pH plays a pivotal role in countless chemical processes, reactions, and biological functions. From regulating the acidity of soil in plant growth to stabilizing biochemical pathways within living organisms, pH has a profound impact on the stability and functioning of various systems.
One of the key mechanisms for controlling pH is through the use of buffer systems. These systems have the remarkable ability to resist changes in pH when small amounts of acids or bases are added.
The Origin of the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical expression that underpins the behavior of buffer systems. It was developed by two brilliant scientists, Lawrence Joseph Henderson and Karl Albert Hasselbalch, in the early 1900s.
Imagine a weak acid, such as acetic acid, dissolved in water. This acid partially dissociates into its constituent ions, hydrogen ions (H+) and acetate ions (CH3COO-). The extent of this dissociation is determined by its dissociation constant (Ka).
The Henderson-Hasselbalch equation quantifies this relationship:
pH = pKa + log ([A-]/[HA])
where:
- pH is the measure of acidity
- pKa is the negative logarithm of the dissociation constant (pKa = -logKa)
- [A-] is the concentration of the conjugate base (acetate ions)
- [HA] is the concentration of the weak acid (acetic acid)
This equation reveals that the pH of a buffer system is directly related to the pKa of the weak acid and the relative concentrations of its conjugate base and acid. It provides a powerful tool for predicting and adjusting pH in various applications.
Practical Significance of Buffer Systems
Buffer systems are essential in a wide range of biological and industrial settings, where maintaining a specific pH range is crucial. For instance:
- Biological systems: Blood has a complex buffer system that tightly regulates its pH within a narrow range. Deviations from this range can lead to severe consequences.
- Industrial processes: Buffers are used in food production, chemical manufacturing, and metallurgy to ensure optimal conditions for specific reactions.
Understanding the principles of buffer systems is therefore essential for maintaining pH homeostasis and optimizing processes in various disciplines.
Explain the key terms: pH, pKa, and buffer component concentrations.
Understanding the Secrets of Buffer Systems
In the realm of chemistry, buffers play a crucial role in maintaining the delicate balance of pH. Let’s dive into the captivating world of weak acid buffer systems, exploring the key terms that govern their behavior.
pH: The Measure of Acidity and Basicity
Imagine a symphony orchestra, where pH is the conductor, directing the balance of acidity and basicity. It’s a numerical scale that ranges from 0 to 14, with 7 representing neutrality.
pKa: The Acid’s Strength
Every weak acid has a unique characteristic called pKa, which measures its strength as an acid. The lower the pKa, the stronger the acid. It’s like the acid’s fingerprint, revealing its personality in the chemical world.
Buffer Component Concentrations: The Ingredients of Stability
A buffer system is like a culinary masterpiece, where the ingredients are the concentrations of the weak acid and its conjugate base. These components work in concert to maintain a stable pH, even when small amounts of acid or base are added.
By understanding these key terms, you’ll unlock the secrets to unraveling the behaviors of weak acid buffer systems. Now, let’s explore how these concepts come together to shape the fascinating world of pH.
Understanding Buffer Systems: The Key to pH Stability
Buffer systems are like the silent guardians of our world, protecting and maintaining a stable pH environment in both biological systems and industrial processes. They work tirelessly behind the scenes to prevent drastic changes in acidity or alkalinity, which can disrupt delicate chemical reactions and biological processes.
Weak acid and weak base buffer systems are the most common types, and they consist of a weak acid or base paired with its conjugate base or acid. The pH of a buffer system is influenced by the relative amounts of the acid and its conjugate base. When the pH is low, more acid is present, while a high pH indicates a higher concentration of the conjugate base.
The Henderson-Hasselbalch Equation: Unveiling the Secrets of Buffer Systems
The Henderson-Hasselbalch equation is the mathematical key that unlocks the secrets of buffer systems. This equation, named after Lawrence Joseph Henderson and Karl Albert Hasselbalch, allows us to calculate the pH of a buffer system based on its component concentrations and the pKa of the weak acid or base.
The pKa is a constant that represents the strength of the acid or base, with a lower pKa indicating a stronger acid. The Henderson-Hasselbalch equation is:
pH = pKa + log([A-]/[HA])
where:
- pH is the acidity or alkalinity of the buffer system
- pKa is the dissociation constant of the weak acid or base
- [A-] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid or base
Using this equation, we can determine the pH of a buffer system by knowing the pKa and the concentrations of its components. For example, if a buffer system has a pKa of 4.5 and equal concentrations of the conjugate base and weak acid, the pH would be 4.5.
Buffer systems are essential for maintaining a stable pH in many applications. In biological systems, they play a vital role in regulating enzyme activity, maintaining cell structure, and transporting nutrients. In industrial processes, they are used to control the acidity or alkalinity of solutions, prevent corrosion, and enhance product quality.
Connect pH to pKa Using the Henderson-Hasselbalch Equation
In the realm of chemistry, understanding the relationship between pH and pKa is crucial for mastering the intricacies of buffer systems. These systems, like tiny guardians, play a pivotal role in maintaining the delicate pH balance in biological and chemical environments.
At the heart of this relationship lies the Henderson-Hasselbalch equation, a mathematical formula that connects these two important values: pH and pKa. The equation, like a magic decoder ring, allows us to decipher the buffer’s behavior and predict its response to changes in its environment.
The pKa Puzzle
pKa, a logarithmic measure of acidity, provides a sneak peek into the strength of the weak acid component in a buffer system. A lower pKa signifies a stronger acid, while a higher pKa indicates a weaker acid. This understanding lays the foundation for comprehending how pH influences the buffer’s characteristics.
pH and pKa in Conversation
The Henderson-Hasselbalch equation becomes the bridge between pH and pKa, establishing a direct relationship between these two values:
pH = pKa + log([A-]/[HA])
In this equation, [A-] represents the concentration of the conjugate base, while [HA] represents the concentration of the weak acid. By manipulating this equation, we can determine the pH of a buffer system based on its pKa and component concentrations.
Unveiling the Secrets of Buffer Strength
The Henderson-Hasselbalch equation also unravels the secrets of buffer strength, a measure of its ability to resist drastic pH changes. Buffer capacity, an intrinsic characteristic of buffers, is directly influenced by the concentrations of both the acid and conjugate base components. Higher concentrations lead to greater buffer capacity, making the system more resilient to pH fluctuations.
Using the Henderson-Hasselbalch equation as a tool, scientists can effectively tailor buffer systems to meet specific requirements, ensuring optimal pH control in diverse applications, from regulating acidity in biological processes to maintaining stable conditions in industrial processes.
The Power of Buffers: Understanding Their Capacity and Component Concentration
In the realm of chemistry, buffers hold a critical role in maintaining the delicate balance of pH levels, similar to the way a steady hand balances a delicate scale. These buffer systems, typically composed of weak acids or weak bases, act like chemical guardians, preventing extreme pH shifts by absorbing excess H+ or OH- ions.
The buffer capacity of a buffer system, like a reservoir’s capacity to hold water, determines its ability to resist pH changes. It is directly proportional to the concentrations of the buffer components, the weak acid (HA) and its conjugate base (A-). Higher concentrations of HA and A- result in a larger buffer capacity, providing a greater ability to neutralize added acids or bases.
Imagine a scenario where you accidentally spill a drop of strong acid into a buffer solution. The buffer acts like a sponge, absorbing the excess H+ ions and preventing a drastic decrease in pH. Conversely, adding a drop of strong base would cause the buffer to absorb the OH- ions, mitigating the increase in pH.
The optimal buffer performance occurs when the concentrations of HA and A- are roughly equal, providing the greatest buffer capacity. However, as the ratio of HA to A- shifts, the buffer capacity decreases. This relationship can be visualized as a bell-shaped curve, with the maximum buffer capacity occurring at equal concentrations.
Understanding the role of buffer capacity and component concentrations is crucial for designing and optimizing buffer systems in various applications, including pH control in biological processes, industrial reactions, and environmental monitoring. By carefully selecting and adjusting buffer components, chemists can tailor buffer systems to meet specific pH requirements and ensure the stability and functionality of pH-sensitive systems.
Explain how the Henderson-Hasselbalch equation applies to weak base buffers.
Weak Base Buffer Systems: Understanding the Henderson-Hasselbalch Equation
In the realm of buffer systems, weak bases also play a crucial role in pH control. Just like weak acid buffers, the Henderson-Hasselbalch equation applies to weak base buffers, providing a powerful tool for understanding their behavior.
The Henderson-Hasselbalch equation for a weak base buffer is written as:
pH = pKb + log([A-] / [HA])
where:
- pH is the measure of acidity or alkalinity
- pKb is the negative logarithm of the dissociation constant of the weak base
- [A-] is the concentration of the conjugate acid of the weak base
- [HA] is the concentration of the weak base
This equation reveals that the pH of a weak base buffer is directly related to the pKb and the relative concentrations of the conjugate acid and the weak base.
When the concentration of the conjugate acid is higher than the concentration of the weak base, the pH will be lower, indicating a more acidic environment. Conversely, when the concentration of the weak base is higher than the concentration of the conjugate acid, the pH will be higher, indicating a more basic environment.
This understanding is essential for manipulating the pH of a weak base buffer. By adjusting the concentrations of the conjugate acid and the weak base, we can fine-tune the pH to meet the specific requirements of a given application.
Real-World Applications
Weak base buffers find widespread use in various industries and biological systems. For instance, they play a vital role in:
- Blood: The bicarbonate buffer system in our blood helps maintain a stable pH within a narrow range, crucial for optimal bodily functions.
- Pharmaceuticals: Weak base buffers are used to control the release of drugs in the body, ensuring their effectiveness and minimizing adverse effects.
- Industrial Processes: Buffer systems are employed in chemical reactions to optimize pH conditions, enhancing efficiency and preventing undesirable side reactions.
Understanding the Henderson-Hasselbalch equation for weak base buffers empowers us to control pH with precision, ensuring optimal conditions for a wide range of applications.
Understanding Weak Base Buffers
In the world of chemistry, buffer systems play a crucial role in maintaining the delicate balance of pH levels. One type of buffer, known as a weak base buffer, involves the interaction between a weak base and its conjugate acid.
The Henderson-Hasselbalch equation is our guide to understanding these buffers. This equation allows us to calculate the pH of a weak base buffer using the pKa of its conjugate acid and the concentrations of the buffer components.
The key to determining the pH of a weak base buffer lies in understanding the relative amounts of the weak base and its conjugate acid. As the concentration of the weak base increases, the pH of the buffer also increases. Conversely, as the concentration of the conjugate acid increases, the pH decreases.
The pH of a weak base buffer typically falls within a range of 1 unit above or below the pKa of its conjugate acid. This range represents the buffer’s effective buffering capacity, where it can effectively resist pH changes when small amounts of acids or bases are added.
Buffer systems are indispensable in maintaining the stability and functionality of biological organisms and industrial processes. By regulating pH levels, they protect sensitive molecules from degradation and ensure optimal conditions for various biochemical reactions.
Understanding pH Manipulation in Buffer Systems
Adjusting pH: A Balancing Act
Buffer systems are resilient, maintaining their pH within a narrow range despite the addition of small amounts of acid or base. However, drastic pH changes can overwhelm their capacity. Understanding how to adjust pH in buffer systems without disrupting their delicate balance is crucial.
Tweaking Buffer Component Concentrations
One method to modulate pH is by altering the concentrations of buffer components, weak acid and its conjugate base. Increasing the concentration of the weak acid will lower the pH, while increasing the concentration of the conjugate base will raise the pH. This adjustment shifts the equilibrium between the acid and base forms, influencing the pH.
Adding Strong Acids or Bases: A Precision Tool
Another strategy is to introduce strong acids or bases. Adding strong acid will decrease pH, and adding strong base will increase pH. However, caution is necessary as the effect is rapid and precise. Overdoing it can disrupt the buffer system’s capacity.
pH Optimization: Avoiding Extremes
When adjusting pH, it’s essential to respect the buffer capacity, which reflects the buffer’s ability to resist large pH shifts. Exceeding the buffer capacity can lead to drastic pH changes, potentially compromising the system’s function or even causing harm.
Ensuring Optimal Function: Balancing pH
In real-world applications, buffer systems play a critical role in maintaining stable pH levels. In biological organisms, buffer systems regulate intracellular pH, which is essential for enzyme activity and overall cell function. In industrial processes, buffers ensure optimal conditions for chemical reactions and product stability.
By understanding how to manipulate pH in buffer systems, we empower ourselves to optimize their functionality, enabling them to fulfill their vital roles in various scientific and industrial applications.
Buffer Systems: A pH Balancing Act
Imagine your body as a delicate ecosystem, where countless chemical reactions occur simultaneously. The pH balance of your cells and fluids is crucial for these reactions to function properly. Buffer systems are like chemical guardians, maintaining a stable pH environment that’s essential for life.
Understanding Buffer Systems
Buffer systems consist of a weak acid and its conjugate base or a weak base and its conjugate acid. The pH of a buffer solution is influenced by the relative amounts of these components. When you add a small amount of strong acid or base to a buffer, it can absorb the excess ions, preventing drastic pH changes.
Henderson-Hasselbalch Equation: Calculating pH
The Henderson-Hasselbalch equation is a mathematical tool used to determine the pH of a buffer solution:
pH = pKa + log([A-]/[HA])
Where:
- pH is the pH of the buffer
- pKa is the dissociation constant of the weak acid
- [A-] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
This equation helps us understand the relationship between pH and pKa and how buffer component concentrations affect pH.
Practical Considerations for Weak Acid Buffers
The buffer capacity of a solution is its ability to resist pH changes. It’s directly proportional to the concentrations of the buffer components. A higher buffer capacity means a more stable pH.
Avoiding Drastic pH Changes
Buffer capacity has its limitations. If you add too much strong acid or base, the buffer system may become overwhelmed and the pH can change dramatically. This can disrupt the sensitive chemical reactions that rely on a stable pH environment.
Real-World Applications of Buffer Systems
Buffer systems are found in virtually every biological organism and play a vital role in industrial processes.
- In the human body, buffer systems regulate pH in blood, urine, and other fluids, ensuring optimal functioning.
- In the food industry, buffers are used to preserve the flavor and quality of foods, while in the pharmaceutical industry, they control the release of active ingredients in medications.
Buffer systems are pH guardians, maintaining a stable chemical environment essential for life. By understanding the principles of buffer systems and their limitations, we can appreciate the delicate balance of our bodies and the importance of avoiding drastic pH changes.
Buffer Systems: Maintaining the Delicate Balance of pH
1. Understanding Buffer Systems
Weak acid and weak base buffer systems play a crucial role in maintaining pH stability. They are composed of a weak acid (or base) and its conjugate base (or acid). When pH changes, these systems shift their composition to counteract the change, ensuring a relatively constant pH.
2. The Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula that quantifies the pH of a buffer system. It relates pH to the pKa of the weak acid (or base) and the concentrations of its components. By plugging in these values, we can calculate the pH and predict how a buffer system will respond to changes.
3. Practical Considerations for Weak Acid Buffers
The Henderson-Hasselbalch equation can help us determine the optimal pH range for a buffer system. Buffers have a limited buffer capacity, which is the amount of acid or base they can absorb before their pH changes significantly. By adjusting the component concentrations, we can optimize the buffer capacity and ensure it functions effectively within the desired pH range.
4. Weak Base Buffer Systems
Weak base buffer systems operate on the same principles as weak acid buffers. The Henderson-Hasselbalch equation can be used to determine their pH as well. By adjusting the concentrations of the weak base and its conjugate acid, we can tailor the buffer system to suit specific pH requirements.
5. pH Manipulation in Buffer Systems
Buffer systems can be manipulated to alter pH. Adding strong acids or bases can shift the equilibrium, causing the pH to increase or decrease, respectively. However, it’s important to consider the buffer capacity and avoid drastic pH changes to prevent buffer exhaustion.
6. Real-World Applications of Buffer Systems
Biological organisms and industrial processes rely heavily on buffer systems to maintain optimal pH levels. In the human body, for instance, blood is buffered to maintain a pH of 7.4, which is essential for cellular function. In industries, buffer systems are used to neutralize wastewater, maintain pH levels in chemical reactions, and preserve the stability of dyes and textiles.
Buffer systems are indispensable tools for controlling and maintaining pH in a wide variety of applications. By understanding the principles of buffer systems and using the Henderson-Hasselbalch equation, we can design and optimize buffers to meet specific pH requirements. From balancing the delicate pH of the human body to facilitating industrial processes, buffer systems play an invaluable role in ensuring stability and optimal functioning.
Buffer Systems: Guardians of pH Stability and Optimal Functioning
Imagine your body as a symphony orchestra, with each component playing a crucial role in creating a harmonious performance. Among these components are buffer systems, the unsung heroes that ensure the pH balance necessary for the orchestra to play flawlessly.
pH: The Master Conductor
The pH of a solution, measured on a scale from 0 to 14, determines its acidity or alkalinity. Optimal pH ranges are essential for biological processes, industrial reactions, and even our own well-being.
Buffer Systems: The pH Stabilizers
Buffer systems are like the sound engineers of the body. They prevent drastic pH changes by absorbing excess H+ ions (acid) or OH- ions (base). This ensures that the pH remains within a narrow range, regardless of internal or external factors.
Importance for Biological Organisms
In the human body, buffer systems protect vital proteins and enzymes from pH fluctuations that can disrupt their function. For example, the blood’s bicarbonate buffer system regulates blood pH and provides the necessary environment for proper cell function.
Applications in Industry
In industrial processes, buffer systems play a crucial role in controlling pH levels for optimal chemical reactions, product stability, and equipment longevity.
Manipulation of pH
Scientists and engineers can adjust the pH of buffer systems by altering component concentrations or adding strong acids/bases. However, it’s crucial to avoid drastic changes due to the limited capacity of buffers to absorb excess ions.
Buffer systems are the unsung heroes of pH stability, ensuring optimal functioning and stability across biological organisms and industrial processes. As we continue to unravel their importance, we gain a deeper appreciation for the delicate balance that sustains life and drives technological advancements.