Understanding The Differences Between Heat And Temperature: A Comprehensive Guide
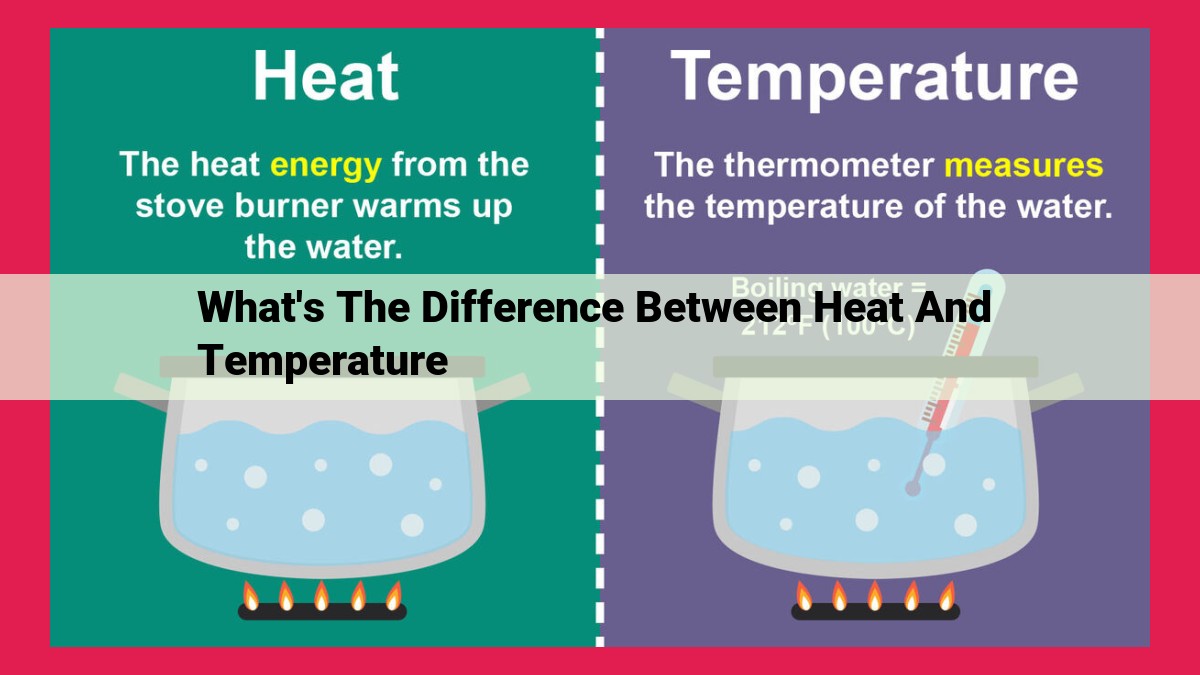
Heat, a form of energy transfer, flows from high to low temperature gradients. It involves the movement of thermal energy, increasing entropy in the system. Temperature, on the other hand, measures the average kinetic energy of molecules in a system. It is a scalar quantity indicating the level of molecular motion, and its equilibrium implies no net heat exchange. While heat flows due to temperature differences, temperature itself does not flow.
Understanding Heat: A Journey into Energy Transfer
Heat is not a substance or a specific thing but rather a form of energy transfer. Imagine a hot stovetop transferring its thermal energy to a cold pot; this is heat flowing from one object to another. Heat always flows from an area of higher temperature to an area of lower temperature, driven by the natural tendency to reach a state of equilibrium.
This temperature gradient determines the direction of heat flow. Molecules and atoms vibrate faster at higher temperatures, possessing more kinetic energy. As heat flows from a hotter object to a cooler one, the faster-moving molecules slow down, transferring their energy to the slower molecules. This process continues until both objects reach the same temperature, creating thermal equilibrium.
The amount of heat required to raise the temperature of an object by one degree Celsius depends on the object’s specific heat. Different materials have different specific heats, which means it takes varying amounts of heat to change their temperature by the same amount. Water, for example, has a high specific heat, requiring a large amount of heat to raise its temperature.
Finally, heat transfer is closely related to the concept of entropy. Entropy measures the disorder or randomness of a system. As heat flows from a hotter object to a cooler one, the disorder or entropy of the system increases. This means that heat transfer inevitably leads to an increase in randomness and a gradual loss of organized energy.
Defining Temperature: The Keystone of Heat Exchange
Temperature, an abstract concept that measures the average kinetic energy of the molecules within a substance, is fundamental to understanding heat transfer and its myriad effects. As heat flows from regions of higher energy to those of lower energy, temperature differences arise, driving the movement of heat.
At thermal equilibrium, when no heat exchange occurs between objects or systems, their temperatures remain constant. Heat capacity, a property that quantifies the amount of heat required to raise the temperature of a substance by one degree, plays a crucial role in determining temperature resistance. Substances with high heat capacities, like water, store more heat before experiencing significant temperature changes.
Phase transitions, such as melting, freezing, boiling, and sublimation, provide vivid examples of how temperature influences the physical states of matter. As heat is added, the average kinetic energy of molecules increases, leading to phase changes from solid to liquid to gas. Conversely, removing heat reverses these transitions, highlighting the profound impact of temperature on the states of matter.
Units of Measurement in the Realm of Heat and Temperature
When we delve into the world of heat and temperature, it is crucial to establish a common language for quantifying these concepts, much like the units of measurement we use to describe distance or time. In this endeavor, the scientific community has adopted the International System of Units (SI), providing us with standardized units for both heat energy and temperature.
The SI unit of heat energy is the joule (J), named after the renowned physicist James Prescott Joule. One joule is defined as the amount of energy required to perform work equivalent to lifting 1 kilogram against the force of gravity over a distance of 1 meter. In the context of heat, a joule represents the energy transferred between objects due to temperature differences.
Moving on to temperature, its SI unit is the kelvin (K), named after the British physicist and engineer Lord Kelvin. The kelvin is defined based on the absolute zero, which is the point where all molecular motion ceases. Absolute zero is equivalent to -273.15 degrees Celsius or -459.67 degrees Fahrenheit. In this scale, the freezing point of water is approximately 273.15 K, while the boiling point is 373.15 K.
While kelvins are the official SI units for temperature, two other scales are commonly used: degrees Celsius (°C) and degrees Fahrenheit (°F). The Celsius scale is widely used in most countries around the world, with 0 °C representing the freezing point of water and 100 °C representing the boiling point. The Fahrenheit scale is primarily used in the United States, where 32 °F corresponds to the freezing point of water and 212 °F to the boiling point.
These units of measurement serve as fundamental tools for scientists, engineers, and researchers to accurately measure, quantify, and communicate heat and temperature values. They enable precise comparisons, calculations, and the establishment of consistent standards across various fields of study.
The Inseparable Bond between Heat and Temperature
Heat and temperature are often used interchangeably, but they are not the same thing. Heat is a form of energy that flows from a warmer object to a cooler object. Temperature, on the other hand, is a measure of how hot or cold an object is.
Heat Flow and Temperature Differences
Heat flows from areas of higher temperature to areas of lower temperature. The greater the temperature difference, the faster the rate of heat flow. This is why you feel cooler on a windy day, even if the temperature is the same. The wind removes heat from your body more quickly.
Thermal Conductivity and Heat Transfer Rates
The thermal conductivity of a material is a measure of how well it conducts heat. Materials with high thermal conductivity, such as metals, transfer heat quickly. Materials with low thermal conductivity, such as wood, transfer heat slowly.
The thickness of a material also affects its ability to conduct heat. A thicker material will conduct heat more slowly than a thinner material.
The Interplay of Heat and Temperature
Heat and temperature are closely related. Heat flow causes temperature differences, and temperature differences drive heat flow. This interplay is essential for many processes, such as cooking, weather forecasting, and manufacturing.
By understanding the relationship between heat and temperature, we can control and harness these forces to improve our lives and the world around us.
Measuring Heat and Temperature
Calorimeters: Capturing Energy Exchange
When we investigate the transfer of heat, calorimeters become our trusted tools. These meticulously designed apparatus track the heat released in chemical reactions or absorbed by substances during temperature changes. By meticulously measuring the change in temperature and accounting for the specific heat of the material, calorimeters provide an accurate measure of heat flow.
Thermometers: Guardians of Temperature
Thermometers, ubiquitous and indispensable, serve as our sentinels for temperature measurement. Their ingenious designs exploit the expansion and contraction of fluids or solids, like mercury or alcohol, to indicate temperature changes. By calibrating these thermometers against defined temperature scales, we can accurately gauge the thermal state of our surroundings.
Thermal Imaging: Unveiling Temperature Variations
Thermal imaging techniques offer a revolutionary window into temperature distributions. These advanced systems utilize infrared sensors to capture thermal radiation emitted by objects, painting a vivid picture of temperature variations. This unparalleled visualization empowers us to detect subtle defects, diagnose medical conditions, and even scrutinize environmental patterns.
The Effects of Heat and Temperature
In the realm of chemistry and physics, heat and temperature play a pivotal role, shaping the properties of matter and driving countless processes. Let’s explore some of the profound effects that heat and temperature exert on our world:
Temperature Changes and Heat Transfer
Heat transfer, the movement of heat between objects or systems, can lead to significant temperature changes. Imagine a cold winter’s day when you step into a warm room. Heat flows from the warm room into your body, raising your temperature and making you feel comfortable. Conversely, on a hot summer day, heat flows from your body into the cooler surroundings, leaving you feeling overheated.
Phase Transitions and Physical States
Heat also influences phase transitions, where substances change from one physical state to another. When you heat ice, it melts into water, and with further heating, the water boils and transforms into vapor. These phase changes are driven by an increase in the average kinetic energy of molecules. As the temperature rises, molecules move faster and become more likely to overcome intermolecular forces, causing the substance to change its physical state.
Chemical Reactions and Temperature Sensitivity
Chemical reactions are highly sensitive to temperature. The rate of a chemical reaction typically increases with increasing temperature. This is because higher temperatures provide more kinetic energy to the reactants, enabling them to overcome the activation energy barrier and react more quickly. For example, the cooking of food involves a series of chemical reactions that are accelerated by the heat of the oven or stovetop.
Heat and temperature are fundamental concepts that permeate our lives and the world around us. They govern changes in the physical states of matter, influence the rates of chemical reactions, and drive countless processes in both natural and technological systems. Understanding the effects of heat and temperature empowers us to control and harness these powerful forces for a wide range of applications, from heating and cooling our homes to cooking food and generating electricity.
Applications of Heat and Temperature: Unraveling the Power of Energy
In our daily lives, heat and temperature play a ubiquitous role, shaping our experiences and influencing countless aspects of our world. From the warmth of our homes to the delicious flavors of our food, the understanding and application of heat have revolutionized our lives.
Heating and Cooling: Controlling the Comfort Zone
One of the most tangible applications of heat is in the realm of temperature control. Heating systems, utilizing furnaces, boilers, or heat pumps, provide warmth during chilly winters, ensuring our comfort and well-being. Conversely, cooling systems, employing air conditioners, fans, or evaporative coolers, bring relief during sweltering summers, maintaining an optimal indoor environment.
Cooking: The Art of Heat Transformation
Heat is an essential element in the art of cooking. Food preparation relies heavily on manipulating heat to transform raw ingredients into delicious meals. Whether roasting, frying, or baking, the judicious application of heat unlocks flavors, tenderizes textures, and creates the culinary masterpieces we savor.
Electricity Generation: Harnessing the Power of Heat
The massive-scale generation of electricity often involves the conversion of heat into other forms of energy. Burning fossil fuels releases enormous amounts of heat, which is then used to drive turbines that generate electricity. This process, while convenient, highlights the challenges of sustainable energy production.
Weather Forecasting: Predicting Temperature Trends
Understanding heat and temperature is crucial for accurate weather forecasting. Meteorologists collect and analyze temperature data to predict weather patterns, from impending storms to seasonal shifts. These forecasts help us prepare for extreme weather events and make informed decisions about our daily activities.
Medical Diagnosis: Fever and Beyond
In the medical field, temperature plays a significant role in diagnosis. Fever, a heightened body temperature, is an indicator of infection or illness. Thermometers, essential tools in medical practice, enable healthcare professionals to monitor and assess patient temperatures accurately.
Scientific Research: Heat as a Catalyst for Discovery
Scientific research heavily relies on the study of heat and temperature to understand various phenomena. From the study of thermodynamics to the analysis of chemical reactions, heat provides insights into the fundamental workings of the universe.
Heat and temperature are fundamental aspects of our existence, impacting everything from our comfort to our health and the advancement of science. By understanding and harnessing the power of heat, we have unlocked countless possibilities that continue to shape our world.