Unraveling Heat’s Impact: Thermal Expansion, Diffusion, Brownian Motion, And Heat Transfer
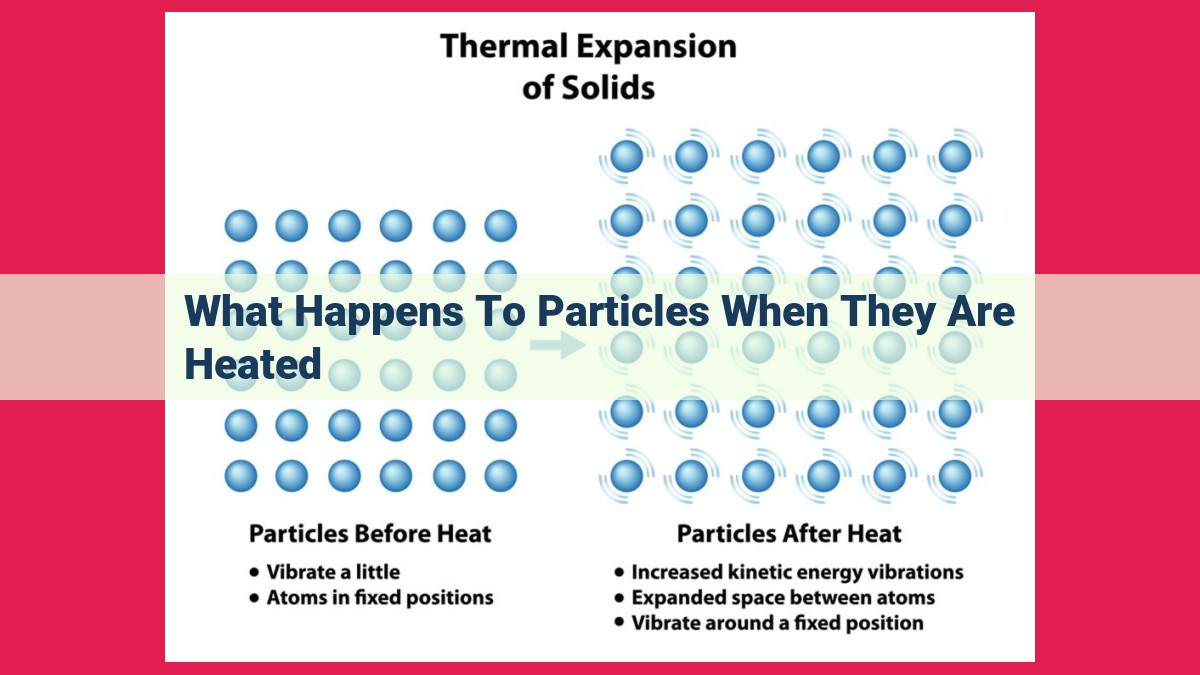
When heated, particles gain kinetic energy, causing them to move faster and accelerate more. This increased motion leads to thermal expansion, where substances increase in volume or length. Additionally, diffusion is enhanced as particles spread from areas of high concentration to low concentration more rapidly. Brownian motion, the random movement of particles, also increases in frequency and amplitude. Heat transfer can occur through convection, involving fluid movement, and radiation, where infrared waves are emitted by heated particles.
Heat: Unraveling Its Effects on Particles
Imagine a bustling city where tiny inhabitants, the particles, are constantly in motion. This energy they possess, aptly named kinetic energy, defines their speed and movement. But what happens when an invisible force, heat, enters this metropolis?
Heat, the transfer of thermal energy, acts like a catalyst, amplifying the particles’ kinetic energy. As this energy surges through them, they become more energetic, zooming around more rapidly with increased acceleration. This heightened activity marks the profound influence of heat on particles.
Temperature as a Measure of Kinetic Energy
- Define temperature as the average kinetic energy of particles.
- Explain the relationship between heat and temperature, where adding heat increases temperature.
Temperature: The Yardstick of Particle Motion
Imagine you’re at the playground and the children are bouncing on a trampoline. Which child would you say is having the most fun? The one that’s jumping the highest, right? In the world of particles, temperature is like a measure of that bounce. It tells us how energetic the particles are, or how fast they’re moving.
You see, heat is like adding energy to the particles, making them excited and eager to party. As particles get hotter, their average kinetic energy rises. Picture the trampoline kids getting energized and jumping even higher. This increased movement is what we feel as temperature. It’s a way of measuring the intensity of particle motion.
So, when you add heat to something, you’re actually giving its particles a boost of energy, making them bounce around more vigorously. And just like the trampoline kids, the more heat you add, the higher the temperature climbs.
Thermal Expansion: Heat’s Impact on Volume and Shape
When you heat an object, a fascinating phenomenon occurs known as thermal expansion. It’s as if the particles within the object start dancing more vigorously, jostling and pushing against each other, causing the object to increase in volume or length. This behavior is a direct consequence of the increased kinetic energy the particles gain from the heat, making them move faster and colliding more frequently.
Understanding thermal expansion is crucial in various applications. For instance, thermometers, the devices we use to measure temperature, rely on the thermal expansion of a liquid (typically mercury) contained within a glass tube. As the temperature rises, the liquid expands, causing it to rise in the tube, indicating a higher temperature.
Another interesting application of thermal expansion is in bimetallic strips. These strips are composed of two different metals bonded together. When heated, the metals expand at varying rates, causing the strip to bend. This bending effect is utilized in thermostats and circuit breakers to regulate temperature and protect against electrical overloads.
In summary, thermal expansion is a fundamental concept demonstrating the influence of heat on the behavior of particles within a substance. It has practical applications in various fields, including temperature measurement, temperature control, and the design of mechanical systems.
Diffusion: The Movement from High to Low Concentration
In the realm of particles, diffusion reigns supreme. It’s the process by which particles spread out, moving from areas where they’re densely packed to those with fewer companions. Imagine a crowded room, filled with energetic individuals; diffusion would be the force that gradually disperses the crowd, allowing people to move more freely.
Heat plays a pivotal role in this diffusion dance. When you introduce heat to the system, it’s like adding fuel to the fire. The particles gain more kinetic energy, which translates to more movement and faster speeds. This increased energy gives the particles the impetus to break free from their densely populated neighborhoods and explore the less crowded areas.
As a result, diffusion accelerates under the influence of heat. The particles have more drive to spread out, creating a more uniform distribution. This phenomenon has numerous applications in the world around us. For instance, it’s the force behind the spread of scents, as odor particles diffuse from their source, reaching our noses and tantalizing our senses.
Diffusion is also essential in biological processes. In our bodies, oxygen and nutrients diffuse from the bloodstream into cells, providing them with the sustenance they need to thrive. Conversely, waste products diffuse out of cells and into the bloodstream, to be eventually expelled from the body.
So, the next time you experience the tantalizing aroma of freshly baked bread or feel the cool breeze on your skin, remember the power of diffusion. It’s the dance of particles, driven by heat, that connects us to the world around us.
Brownian Motion: Unveiling the Dance of Tiny Particles
In the realm of science, microscopic particles engage in an intriguing dance known as Brownian motion. Imagine a troupe of tiny dancers, suspended in a gentle fluid, performing a chaotic ballet. This seemingly random movement holds fascinating secrets about the nature of matter and heat.
As we crank up the temperature, the particles become more energized, like lively dancers with an insatiable thirst for motion. Their kinetic energy skyrockets, propelling them to move with greater speed and abandon. This increased agitation amplifies the frequency and amplitude of their dance, creating a captivating spectacle.
Unlocking the Mystery of Particle Behavior
Brownian motion, discovered by the brilliant physicist Robert Brown, provides a window into the inner workings of matter. By observing the erratic trajectories of particles suspended in a fluid, scientists can deduce their size, shape, and even their interactions with the surrounding liquid.
Moreover, Brownian motion has revolutionized research in various fields. In biology, it helps unravel the secrets of cellular processes. In chemistry, it provides insights into the collision dynamics of molecules. And in physics, it allows researchers to probe the fundamental properties of particles.
Practical Applications of Brownian Motion
The principles behind Brownian motion extend beyond the confines of the laboratory. In the world of engineering, this chaotic dance has inspired ingenious inventions. Take, for example, the Brownian motor, a tiny engine that harnesses the random motion of particles to produce electricity without moving parts.
Brownian motion is a captivating phenomenon that reveals the intricate relationship between heat and particle behavior. By studying this dance of tiny particles, scientists have gained invaluable knowledge about the nature of matter and unlocked practical applications that shape our world. From exploring the depths of cells to powering innovative devices, Brownian motion continues to inspire and enchant the scientific community.
Convection: The Dance of Heat in Moving Fluids
What is Convection?
Convection is a mesmerizing dance of heat transfer, where the movement of fluids (liquids or gases) carries warmth along like an invisible ballet. As you heat a fluid, its tiny particles start buzzing with more energy, causing them to expand and become less dense. This sets the stage for an enchanting move: the buoyant rise.
The Buoyant Rise
Imagine a pot of simmering water. As the water heats up at the bottom, its particles gain energy and expand, becoming less dense. This creates a region of lighter water that rises gracefully towards the surface. As the buoyant water ascends, it carries heat along like a thermal escalator, distributing the warmth throughout the liquid.
The Global Symphony of Convection
Convection isn’t just a captivating dance in your kitchen; it’s a grand symphony that orchestrates the Earth’s weather patterns and the distribution of heat in our vast oceans and atmospheres. When air near the Earth’s surface is heated, it rises, creating updrafts that drive wind currents and form clouds. In the oceans, convection currents circulate warm water from the tropics to the poles, distributing heat and nutrients to marine life.
Applications of Convection
The dance of convection has numerous applications in our daily lives and beyond. In radiators and baseboards, convection currents carry warm air around rooms, providing comfort and warmth. In oceanic currents, convection plays a crucial role in regulating global temperatures and supporting marine ecosystems. And in industrial processes, convection is harnessed to cool down equipment and circulate fluids for optimal efficiency.
Convection is an enchanting force that governs the movement of heat in fluids. From the gentle stirring of a simmering pot to the grand symphony of weather patterns, convection shapes our world in countless ways. Understanding its principles allows us to appreciate the subtle yet profound role it plays in the intricate dance of nature.
Radiation: Heat Transfer through the Unseen Realm
In the vast tapestry of heat transfer, radiation stands apart as a mysterious and invisible force. Unlike conduction and convection, which require the movement of matter, radiation weaves its magic through the ethereal realm of electromagnetic waves.
As particles dance with energy, they emit these waves, which carry heat across vast distances. Like tiny beacons, they emit infrared radiation, invisible to our eyes, but warmly felt on our skin.
Heat, measured as the kinetic energy of particles, fuels the dance of radiation. The more energetic the particles, the more intense the radiation they emit. This phenomenon governs the heat felt from the sun’s rays, which reach us through the vacuum of space.
In the cosmic vacuum, where matter is scarce, radiation reigns supreme as the primary mode of heat transfer. Stars, the luminous orbs that dot the night sky, emit their brilliance through radiation, spreading warmth and energy throughout the galaxy.
Moreover, radiation plays a crucial role in Earth’s climate. The Earth’s surface absorbs sunlight during the day, emitting infrared radiation back into the atmosphere at night. This process, known as radiative cooling, helps regulate Earth’s temperature, maintaining a habitable environment for life to flourish.
From the celestial dance of stars to the subtle warming of our homes, radiation serves as a silent yet potent force, shaping the way heat travels through our world.