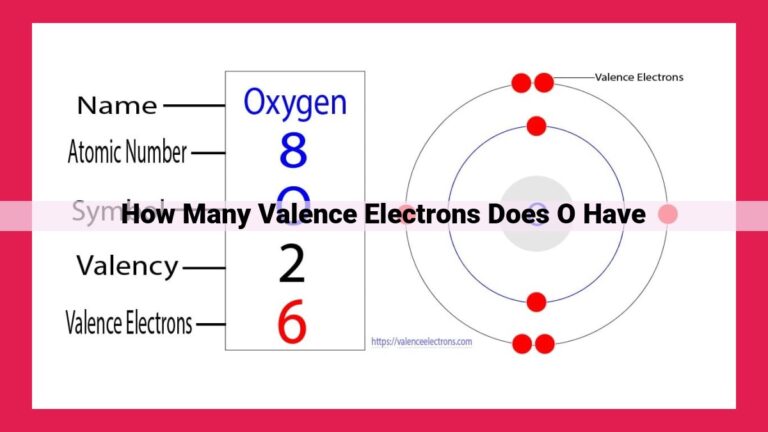
Halogens: Properties, Reactivity, And Applications In Chemistry
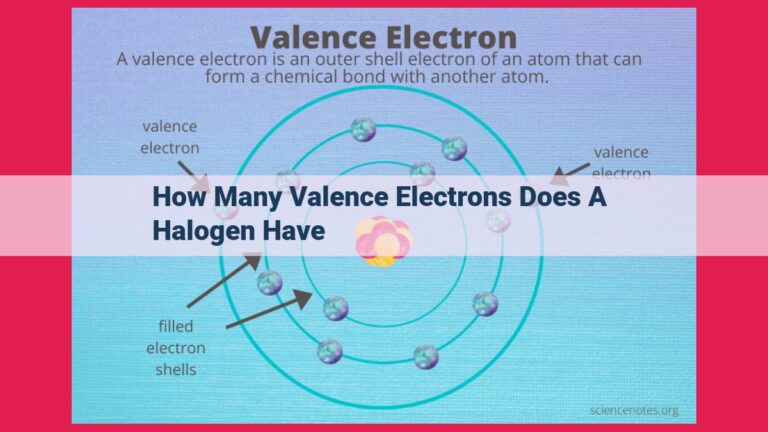
Halogens, located in Group 17 of the periodic table (F, Cl, Br, I, At), possess seven valence electrons, making them highly reactive. Valence electrons, the outermost electrons of an atom, determine its chemical properties. Halogens’ seven valence electrons, represented in their electron configurations, allow them to readily gain an electron (oxidation state of -1) or form covalent bonds with nonmetals, seeking a stable octet of valence electrons. This reactivity makes halogens effective in applications such as disinfectants (e.g., chlorine) and in the synthesis of various compounds.
Unveiling the Secrets of Valence Electrons: The Key to Understanding Halogens
In the realm of chemistry, the concept of valence electrons holds the key to unraveling the intriguing behavior of elements. These electrons, the outermost dance partners of an atom, play a pivotal role in shaping the chemical properties that define each element. Join us on a captivating journey as we delve into the fascinating world of valence electrons, focusing specifically on the enigmatic group of halogens.
The Dance of Valence Electrons
Imagine electrons as tiny celestial bodies orbiting the nucleus of an atom, each with its own energy level. The outermost level, the energy frontier, is where the valence electrons reside. These electrons participate in the chemical tango, determining the element’s ability to interact with others.
Halogens: The Seven Sisters
On the periodic table, nestled in Group 17, resides a family of five elements known as the halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements share a captivating secret: they all possess seven valence electrons.
The Halogen Paradox: Reactivity and Stability
This unique electronic configuration grants halogens an intriguing duality. On one hand, their seven valence electrons make them highly reactive. With a thirst for stability, they readily accept an electron to complete their outer shell, forming ionic bonds with alkali metals. On the other hand, these same valence electrons enable halogens to form covalent bonds with nonmetals, sharing electrons to achieve a stable configuration.
The Legacy of Valence Electrons
The imprint of valence electrons is etched into the chemical tapestry of halogens. Their reactivity makes them indispensable in everyday applications: chlorine purifies water, fluorine strengthens toothpaste, and iodine is used as an antiseptic. Their tendency to form covalent bonds underpins the existence of countless organic molecules, the building blocks of life itself.
So, dear reader, as you embark on your chemical explorations, remember the profound significance of valence electrons. They are the invisible puppeteers, orchestrating the dance of elements and shaping the world of chemistry.
Delving into the Realm of Halogens: Sentinels of Reactivity
As we embark on a captivating expedition into the world of chemistry, we encounter a fascinating group of elements known as halogens. These elements, residing in Group 17 of the periodic table, have carved a niche for themselves due to their intriguing electronic configurations and remarkable chemical properties. Meet fluorine, chlorine, bromine, iodine, and astatine, the enigmatic quintet that comprises the halogen family.
What Sets Halogens Apart?
The defining characteristic of halogens lies in their seven valence electrons. These electrons, residing in the outermost shell of their atoms, play a pivotal role in dictating the chemical behavior of halogens. Their eagerness to complete their valence electron octet drives their relentless pursuit of chemical companionship.
Masters of Reactivity
The presence of seven valence electrons bestows halogens with exceptional reactivity. They readily engage in chemical reactions, forming both ionic and covalent bonds. Their ability to form ionic bonds with alkali metals exemplifies their strong electronegativity, while their capacity to forge covalent bonds with nonmetals underscores their versatile chemical nature.
Harnessing the Power of Halogens
Due to their high reactivity, halogens find myriad applications in our daily lives. Fluorine, for instance, enhances the strength of toothpaste and drinking water, while chlorine serves as a potent disinfectant in swimming pools and water treatment facilities. Bromine contributes to the manufacture of flame retardants and photographic materials, while iodine plays a crucial role in thyroid hormone production.
Valence Electrons: The Secret to Halogens’ Reactivity
In the realm of chemistry, valence electrons play a pivotal role in shaping the properties of elements. These electrons, located in the outermost shell of an atom, are the key to understanding why halogens – a fascinating group of elements – are so uniquely reactive.
Halogens’ Home on the Periodic Table
Halogens reside in Group 17 of the periodic table, a special club of elements that share a common trait: seven valence electrons. This electron configuration, like a fingerprint, defines their chemical destiny.
Seven Valence Electrons: A Recipe for Reactivity
Imagine a halogen atom as a small, lively ball with seven electrons buzzing around it like tiny bees. These seven valence electrons are the key to halogens’ remarkable reactivity. They are the gateway to forming both ionic and covalent bonds, the chemical glue that holds atoms together.
Ionic bonds arise when halogens pair up with alkali metals, which have just one valence electron. The halogen atom “borrows” this extra electron, creating a positive ion (the alkali metal) and a negative ion (the halogen). Covalent bonds, on the other hand, form when halogens share their valence electrons with other nonmetallic elements.
Implications of Seven Valence Electrons
The presence of seven valence electrons has profound implications for halogens’ behavior:
- High Reactivity: Halogens are notoriously reactive, eagerly forming both ionic and covalent bonds. This reactivity makes them essential in countless chemical processes, from disinfecting water to producing pharmaceuticals.
- Electron Affinity: Halogens have a strong affinity for electrons, meaning they readily accept extra electrons to form negative ions. This electron affinity explains their role as oxidizing agents, substances that donate electrons to other molecules.
- Diatomic Nature: Halogens exist as diatomic molecules (pairs of atoms) due to their tendency to bond with themselves. This diatomic nature influences their physical properties, such as their low boiling points and high reactivity.
The seven valence electrons of halogens are the key to understanding their exceptional reactivity and chemical versatility. From their role in forming ionic and covalent bonds to their oxidizing ability, these electrons define the unique properties that make halogens indispensable in chemistry.
The Seven Deadly Valence Electrons: Unveiling the Reactivity of Halogens
In the realm of chemistry, valence electrons reign supreme, dictating the personality of elements and their tendency to engage in chemical shenanigans. Among these elements, the halogens stand out as masters of reactivity, thanks to their unique set of seven valence electrons.
Halogens: The Outsiders of Group 17
Nestled in the far right corner of the periodic table, Group 17 hosts the halogen family: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the enigmatic astatine (At). They’re the rebels of the element world, with their atomic number increasing from top to bottom. And just like rebellious teenagers, halogens have an extra dose of something: seven valence electrons.
Valence Electrons: The Key to Reactivity
Valence electrons are like the restless spirits of an atom, eagerly seeking companionship to fill their empty orbitals. The number of valence electrons determines an element’s chemical properties, shaping its bonding preferences and how it interacts with others. And the halogens, with their seven valence electrons, are always looking for a dance partner.
Ionic Bonds: Hand in Hand with Alkali Metals
Alkali metals, the gentlemen of Group 1, have a singular valence electron, eager to give it up. When halogens meet alkali metals, it’s like a match made in ionic heaven. The halogen’s seven valence electrons greedily snatch the lone valence electron from the alkali metal, forming a stable ionic bond. This gives rise to compounds like sodium chloride (NaCl), the backbone of our salty existence.
Covalent Bonds: Sharing the Spotlight with Nonmetals
But halogens aren’t restricted to just alkali metals. They can also share their valence electrons with nonmetals, forming covalent bonds. In these bonds, both atoms contribute electrons to create a strong, covalent partnership. Chlorine and fluorine, for instance, form the deadly chlorine gas (Cl2), the scourge of World War I.
The seven valence electrons of halogens are the driving force behind their high reactivity. They enable halogens to form ionic bonds with alkali metals for a stable existence or covalent bonds with nonmetals for a more complex, dynamic relationship. Understanding the role of valence electrons in halogens is crucial for comprehending their chemical behavior and their fascinating presence in the world around us.