Halogens: Exploring Their Chemistry And Reactivity In Group 17
Halogens, elements in Group 17 of the periodic table, possess seven valence electrons, influencing their chemical behavior. These valence electrons occupy the outermost energy level of halogen atoms and interact with other atoms to form chemical bonds. The high number of valence electrons makes halogens highly reactive, acting as oxidizing agents. Their electronegativity drives their tendency to gain electrons and become negatively charged ions, enabling the formation of ionic compounds.
Halogens: Unveiling the Secret of Reactivity
In the realm of chemistry, there lies a captivating group of elements known as halogens. These reactive elements reside in Group 17 of the periodic table, a gathering of atoms that share an unquenchable thirst for completing their outermost energy levels.
Unveiling the Essence of Halogens
Halogens are an enigmatic bunch, possessing a unique set of characteristics that set them apart from the rest of the chemical elements. They are:
- Vividly colored, ranging from the pale yellow of fluorine to the deep violet of iodine
- Highly reactive, readily forming compounds with other elements, often acting as oxidizing agents
- Excellent conductors of electricity and heat
The Mystery of Valence Electrons
The key to understanding the extraordinary behavior of halogens lies in their valence electrons. These are the electrons that occupy the outermost energy level of an atom, determining its chemical properties. Halogens possess seven valence electrons, an arrangement that shapes their destiny.
Energy Levels Unraveled
Each atom consists of multiple energy levels, which are discrete regions where the electrons reside. The number of protons in the nucleus determines the number of energy levels. For halogens, there are seven energy levels, with the outermost level holding the seven valence electrons.
Valence Electrons: The Architects of Reactivity
The number of valence electrons profoundly influences an element’s chemical reactivity. Halogens, with their seven valence electrons, are eager to acquire an eighth electron to complete their outermost energy level. This relentless pursuit of a stable octet drives their high reactivity, making them potent oxidizing agents.
The Octet Rule: A Guiding Principle
The octet rule dictates that elements achieve stability by acquiring an octet of valence electrons, resembling the electron configuration of noble gases. Halogens are no exception, forming compounds that satisfy this rule. They react with metals to form ionic compounds, where the halogen gains an electron, and with nonmetals to form covalent compounds, where electrons are shared.
The valence electrons of halogens play a pivotal role in shaping their chemical properties. These elements’ relentless pursuit of a stable octet propels their reactivity and drives their distinctive behavior in chemical reactions. Understanding the interplay between valence electrons and energy levels provides a profound insight into the enigmatic world of halogens.
Valence Electrons: The Driving Force Behind Halogen Reactivity
In the realm of chemistry, the concept of valence electrons holds a pivotal position, determining the chemical behavior of elements. Valence electrons are the outermost electrons in an atom, and their number plays a crucial role in shaping the atom’s reactivity. In this article, we delve into the fascinating world of valence electrons and explore their influence on the halogens, the highly reactive elements that reside in Group 17 of the periodic table.
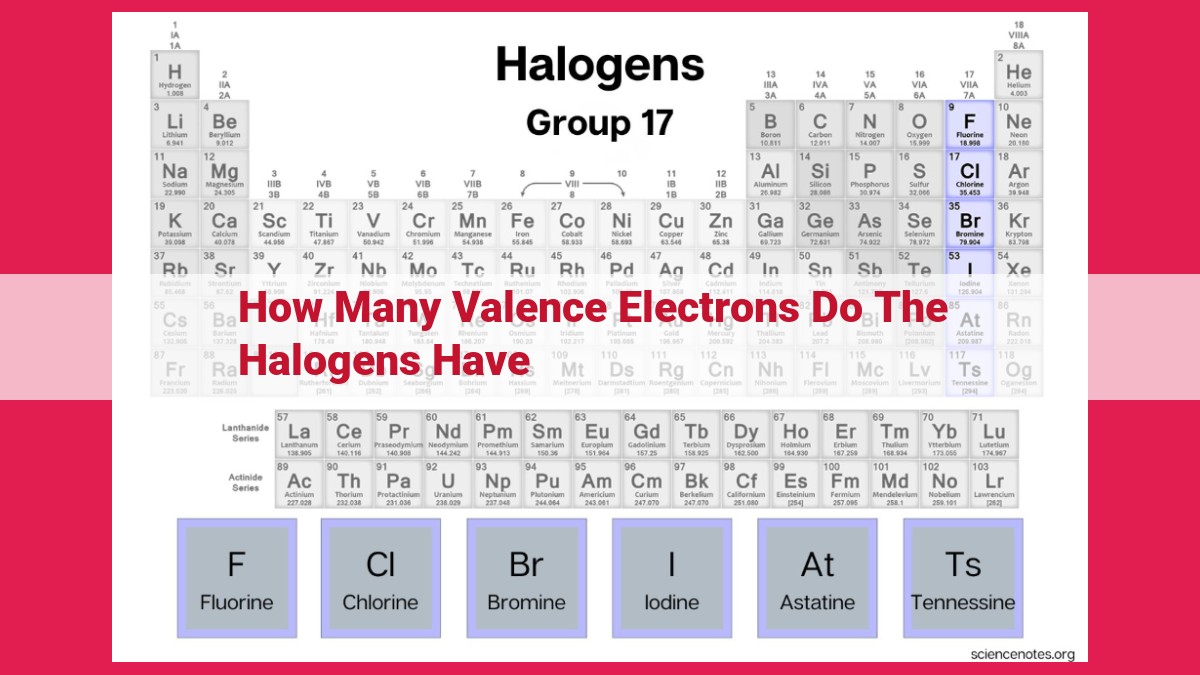
Halogens, including fluorine, chlorine, bromine, iodine, and astatine, are highly reactive elements that readily form compounds with other elements. This remarkable reactivity is attributed to their valence electrons. All halogens have seven valence electrons, which means they are one electron short of a complete outer energy level. This incomplete outer energy level makes halogens eager to gain an electron from other atoms, thereby achieving a more stable electron configuration.
The number of valence electrons in halogens aligns with their position in Group 17 of the periodic table. The arrangement of elements in the periodic table is far from random; it reflects the number of valence electrons they possess. Elements in the same group, such as the halogens, share a common number of valence electrons. This arrangement provides valuable insights into their chemical behavior and provides a framework for predicting their reactivity.
Understanding valence electrons is essential for comprehending the chemical properties of halogens. These highly reactive elements play a vital role in various chemical processes, including biological functions and industrial applications. Their reactivity stems from their tendency to form chemical bonds with other atoms, a process driven by the quest to complete their outer energy levels. By exploring the concept of valence electrons, we gain a deeper appreciation for the intricate workings of the chemical world and the fascinating behavior of the halogens.
Delving into the Energetic World of Halogens
In the realm of chemistry, halogens occupy a captivating position as highly reactive elements. Their unique behavior stems from the intricate dance of their valence electrons, which reside in specific energy levels within their atomic structures.
Unveiling the Structure of Energy Levels
Imagine atoms as miniature solar systems, with a nucleus at the center, akin to the sun, and electrons orbiting it like planets. These electrons inhabit distinct energy levels, similar to the shells surrounding a planet. Each energy level can accommodate a specific number of electrons, and the closer an energy level is to the nucleus, the lower its energy.
The Influence of Protons on Energy Levels
The number of protons within an atom’s nucleus plays a crucial role in determining the energy levels. Each proton carries a positive charge, which exerts an attractive force on negatively charged electrons. The greater the number of protons, the stronger the attraction, pulling electrons closer to the nucleus and creating lower-energy levels.
The Distribution of Valence Electrons in Halogens
Halogens, located in Group 17 of the periodic table, possess seven valence electrons. These electrons occupy the outermost energy level of the atom, which is the highest-energy level. Due to their position on the outermost shell, valence electrons are loosely bound to the nucleus, making them highly reactive.
Halogens strive to achieve a stable electronic configuration known as the “octet rule.” This rule states that atoms are most stable when they have eight valence electrons. In order to attain this stable configuration, halogens readily form chemical bonds with other elements, either by gaining or losing electrons.
The energy levels and valence electrons of halogens are key factors that govern their chemical behavior. Their unique electronic configuration, with seven valence electrons occupying the outermost energy level, makes them highly reactive and eager to form chemical bonds. By understanding the intricate interplay between energy levels and valence electrons, we can unravel the mysteries surrounding the chemical properties of halogens.
Valence Electrons and the Chemistry of Halogens
The Significance of Valence Electrons
Imagine the valency* of atoms as the **chemical handshake they use to interact with each other. These valence electrons determine the atom’s reactivity potential and its ability to form chemical bonds.
Halogens: The Reactive Crowd
Halogens, positioned in Group 17 of the periodic table, are chemical powerhouses with seven* valence electrons in their outer energy level. This **Octet Rule states that atoms are most stable when they have **eight* valence electrons.
Halogens as Oxidizing Agents
The high reactivity of halogens stems from their strong electronegativity and desire to acquire one more electron to achieve a stable octet. As a result, halogens readily oxidize other substances by accepting electrons, causing chemical reactions.
The Halogen-Hydrogen Dance
Take the reaction between fluorine and hydrogen as an example. Fluorine, with its seven valence electrons, reacts with hydrogen, which has one valence electron, to form hydrogen fluoride. In this process, fluorine **gains* one electron from hydrogen, while hydrogen **loses* its electron.
Halogens in Everyday Life
The reactivity of halogens makes them essential in various applications. Chlorine*, for instance, is widely used to **disinfect water and in swimming pools. Bromine*, another halogen, finds its niche as a **fire retardant.
In conclusion, the valence electrons of halogens play a pivotal role in their chemical behavior. Their high reactivity as oxidizing agents makes them valuable in both industrial and domestic settings. Understanding these concepts is fundamental to comprehending the fascinating world of chemistry.