Halogens: Unveiling The Chemical Properties And Reactivity Of Group 17 Elements
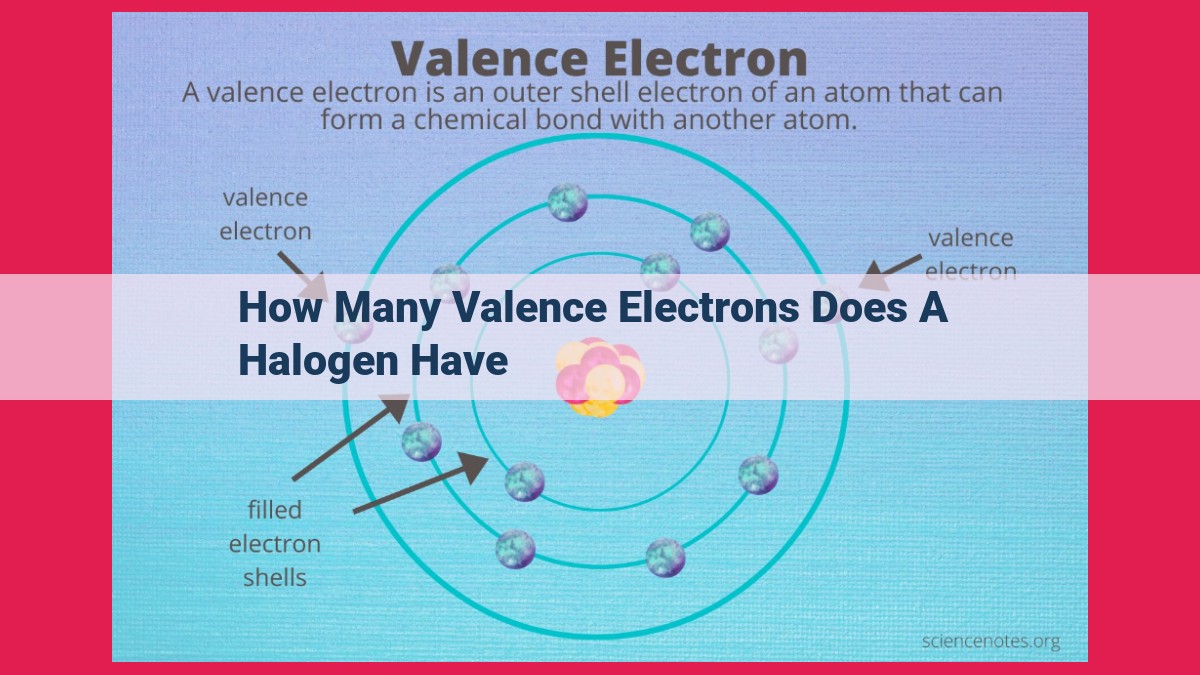
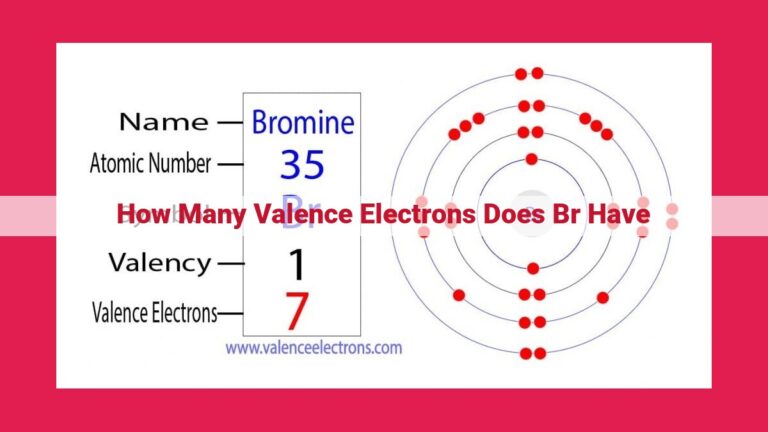
Halogens, elements belonging to Group 17 of the periodic table, consistently possess 7 valence electrons. This uniform electron configuration (ns²np⁵) dictates their high reactivity and electronegativity. The number of valence electrons influences their chemical properties, such as their ability to form covalent bonds and their tendency to accept electrons, making them highly reactive non-metals. This consistent valence electron configuration is crucial in understanding the chemical behavior and properties of halogens.
Valence Electrons: The Building Blocks of Chemistry
In the realm of chemistry, the concept of valence electrons plays a pivotal role. These outermost electrons, residing in the energy level farthest from the atom’s nucleus, hold significance beyond their mere presence. They orchestrate the chemical properties of elements, paving the way for the reactions that shape our world.
The number of valence electrons is a crucial factor in determining an element’s chemical behavior. This notion is especially evident in the periodic table, where elements are arranged based on their increasing atomic number and, in turn, their valence electron configuration. As one traverses the periodic table from left to right, the number of valence electrons increases, leading to predictable trends in chemical properties.
For instance, metals on the left-hand side of the periodic table tend to have a low number of valence electrons and readily give them up, forming positive ions. On the other hand, non-metals, located on the right-hand side, have a higher number of valence electrons and exhibit a strong tendency to gain electrons, becoming negative ions.
Chemical Properties of Halogens: Unveiling the Reactive Essence
Halogens, a family of elements residing in Group 17 of the periodic table, possess a captivating blend of reactivity and electronegativity, making them essential players in a myriad of chemical reactions. Their unique properties stem from their valence electrons, the outermost electrons in their atomic structure that dictate their chemical behavior.
Reactivity Unleashed
Halogens are renowned for their high reactivity, eager to form new bonds with other elements. This reactivity is a consequence of their incomplete valence electron shells. With seven valence electrons, halogens strive to attain a stable, octet configuration by either gaining or sharing electrons. This insatiable desire for electron balance fuels their reactivity, leading to the formation of diverse chemical compounds.
Electronegativity at Play
Electronegativity refers to an element’s ability to attract and hold electrons. Halogens boast high electronegativity, indicating their strong affinity for electrons. This characteristic stems from their small atomic size and high nuclear charge, which creates a strong electrostatic force that attracts electrons towards the halogen nucleus.
Comparative Chemistry
The chemical properties of halogens vary subtly as one moves down the periodic table. Fluorine, the lightest halogen, is the most reactive and electronegative due to its compact size and highest nuclear charge. As one descends the group to chlorine, bromine, and iodine, reactivity and electronegativity decrease. This trend is attributed to increasing atomic size and decreasing nuclear charge, which weakens the electrostatic attraction for electrons.
In conclusion, halogens’ chemical properties, including their reactivity and electronegativity, are intricately linked to their valence electrons. These properties govern their ability to form chemical bonds, participate in reactions, and exhibit distinct chemical behavior. Understanding the influence of valence electrons on halogen chemistry is crucial for comprehending the diverse roles these elements play in the chemical world.
Valence Electron Trends in Halogens: A Consistent Pattern
In the periodic table, the halogens occupy Group 17, known as the “reactive group.” This strategic positioning holds the key to understanding their unique chemical properties. The halogens, comprising fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), share a striking similarity – their valence electron configuration.
Valence electrons, the outermost electrons in an atom, play a crucial role in determining chemical behavior. For halogens, their valence electron configuration remains constant throughout the group. Each halogen possesses seven valence electrons, arranged in the outermost energy level. This uniformity in valence electron configuration translates into predictable and consistent chemical properties among the halogens.
Their shared valence electron configuration accounts for their high reactivity and electronegativity. The presence of seven valence electrons makes halogens eager to gain an electron and achieve a stable noble gas configuration. This electron-hungry nature drives their strong reactivity with other elements, forming stable compounds.
Additionally, the consistent number of valence electrons within the halogen group influences their electronegativity, a measure of an atom’s ability to attract electrons. The closer an element is to fluorine, the more electronegative it becomes. This gradual increase in electronegativity from iodine to fluorine reflects the decreasing atomic radius within the group. As the atomic radius shrinks, the nucleus exerts a stronger pull on the valence electrons, making them less willing to participate in chemical bonding.
In conclusion, the halogens’ consistent valence electron configuration, a result of their position in Group 17, dictates their chemical properties. Their seven valence electrons make them highly reactive and electronegative, while the gradual increase in electronegativity within the group highlights the influence of atomic radius on electron attraction. Understanding these trends is essential for comprehending the chemistry of these fascinating elements.
Electron Configuration of Halogens: Unraveling the Structural Symphony
Embark on a captivating journey into the realm of chemistry, where we unravel the enigmatic electron configuration of halogens – a group of elements that enthrall us with their unique reactivity.
Halogens reside in Group 17 of the periodic table, their common abode. They share a harmonious trait: a consistent electron configuration that sets them apart from the rest. Each halogen possesses seven valence electrons – the pivotal electrons that dance on the outermost energy level, orchestrating their chemical symphony.
This consistent valence electron configuration, ns²np⁵, is the key to understanding the halogens’ remarkable properties. These elements are highly electronegative, eagerly attracting electrons from their dance partners to form bonds. Their high reactivity stems from their desire to complete their valence shell, transforming them into avid electron collectors.
The electron configuration of halogens not only governs their chemical reactivity but also reveals their structural elegance. Halogens typically exist as diatomic molecules, with two atoms tightly bound together. This bond arises from the sharing of valence electrons, a harmonious duet that stabilizes the molecule.
So, dear readers, as we delve into the electron configuration of halogens, we not only unravel the secrets of their chemical properties but also appreciate their intrinsic beauty. These elements are not merely reactive players on the chemical stage; they are intricate dancers, their electron configuration guiding their every move.
The Defining Feature of Halogens: Their Seven Valence Electrons
In the realm of chemistry, valence electrons hold the power to shape the behavior and properties of elements. Among these elements, the halogens stand out with a consistent and defining characteristic: their uniformity in the number of valence electrons.
Understanding Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom, and they determine the chemical properties of the element. The seven elements that make up the halogen group (Group 17) share a remarkable feature—they all possess seven valence electrons. This uniformity plays a crucial role in shaping their reactivity and electronegativity.
Impact on Reactivity
Reactivity refers to an element’s tendency to participate in chemical reactions. Halogens are well-known for their high reactivity, and their seven valence electrons contribute significantly to this behavior. The unfilled valence orbitals of halogens allow them to readily gain electrons, forming stable halide ions (X-). This makes halogens strong oxidizing agents, capable of accepting electrons from other elements and forming chemical bonds.
Influence on Electronegativity
Electronegativity measures an element’s ability to attract electrons towards itself. Halogens are among the most electronegative elements, meaning they have a strong tendency to draw electrons to them. The high electronegativity of halogens results from their seven valence electrons, which contribute to a strong nuclear charge. This charge pulls the electrons closer to the nucleus, giving halogens a high affinity for electrons.
The uniformity in the number of valence electrons among halogens (seven) is a defining characteristic that profoundly impacts their reactivity and electronegativity. This feature makes halogens highly reactive and electronegative, giving them unique chemical properties and making them essential elements in a wide range of applications.