Unveiling The Reactivity Of Halogens: Delving Into The Electronic Configuration Of Group 17 Elements

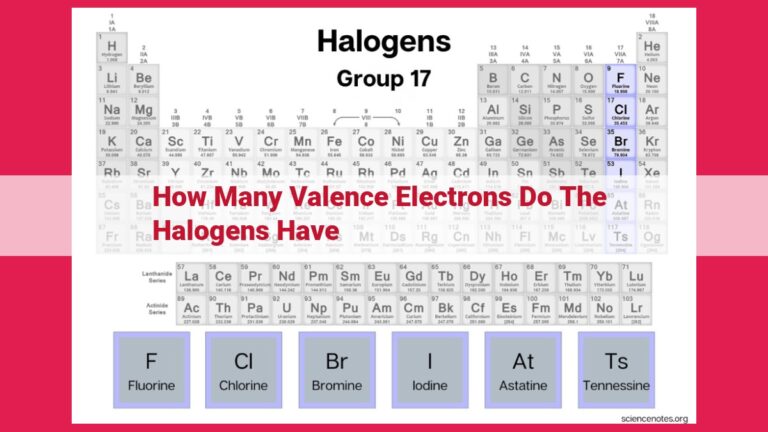
Halogens, a group of elements in Group 17, are highly reactive due to their unique electronic configuration. They have seven valence electrons, which occupy the outermost energy level. This characteristic makes halogens highly electronegative, with a strong tendency to gain or share one electron to achieve a stable configuration. The presence of seven valence electrons determines their chemical properties and explains why halogens are known for their reactivity and ability to form bonds with other elements.
Valence Electrons and Halogens: Unraveling the Chemistry Behind Reactivity
Within the realm of chemistry, understanding the behavior of elements is crucial for comprehending the intricate tapestry of chemical reactions that shape our world. Among these elements, halogens – a family of elements renowned for their high reactivity – stand out as a fascinating subject of study. To grasp the essence of their chemistry, we must delve into the concept of valence electrons.
Valence electrons are the outermost electrons in an atom, playing a pivotal role in determining its chemical reactivity. In the case of halogens, they occupy the seventh energy level. The unique arrangement of these valence electrons grants halogens special chemical properties.
Halogens, residing in Group 17 of the periodic table, are characterized by their nonmetallic nature. Highly reactive, they tend to form diatomic molecules, such as chlorine (Cl₂) and fluorine (F₂). This high reactivity stems from the presence of seven valence electrons, making halogens highly electronegative.
Importance of Valence Electrons: The Key to Halogens’ Reactivity
In the captivating world of chemistry, valence electrons play a pivotal role, shaping the individuality of each element on the periodic table. These electrons, residing in the outermost energy level of an atom, hold the key to understanding the unique chemical behavior of halogens.
The Significance of Valence Electrons
Valence electrons determine an element’s ability to interact with other atoms. They act as gatekeepers, controlling the formation of chemical bonds. Elements that possess similar numbers of valence electrons tend to exhibit similar chemical properties.
Halogens: A Special Group with Seven Valence Electrons
Halogens, the elements that grace Group 17 of the periodic table, stand out from the crowd with their distinctive characteristic: seven valence electrons. This seemingly ordinary number holds immense significance, granting halogens their remarkable chemical reactivity.
With seven valence electrons, halogens are constantly seeking to achieve a stable electron configuration. This drive for stability propels them to readily gain or share electrons, resulting in their characteristic affinity for forming ionic or covalent bonds.
Halogens as Group 17 Elements
- Define halogens as Group 17 elements.
- Discuss their nonmetallic properties, such as being highly reactive and forming diatomic molecules.
- Emphasize that halogens have seven valence electrons, making them highly electronegative.
Halogens: The Reactive Elements with Seven Valence Electrons
In the periodic table, Group 17 holds a special place for a group of elements known as the halogens. These elements—fluorine, chlorine, bromine, iodine, and astatine—share a unique characteristic that sets them apart and plays a pivotal role in their chemical behavior: seven valence electrons.
Valence electrons are the electrons in an atom’s outermost energy level, and they determine how an element interacts with others. For halogens, these seven valence electrons make them highly reactive and prone to forming chemical bonds.
Nonmetallic in nature, halogens are found in various forms, including diatomic molecules like chlorine gas (Cl2) and solid compounds like sodium chloride (NaCl). Their high reactivity makes them essential in various industrial processes, water purification, and the production of everyday items like plastics and disinfectants.
One of the most striking properties of halogens is their electronegativity. This measure of an atom’s ability to attract electrons is exceptionally high for halogens due to their seven valence electrons. This means that halogens have a strong tendency to gain or share electrons to achieve a stable electron configuration.
Fluorine, the most electronegative element, is so reactive that it readily forms compounds with almost all other elements. Chlorine, familiar as a disinfectant in swimming pools, is a highly reactive gas critical in water purification and bleaching processes. Bromine is a liquid element known for its use in photography, while iodine is an essential nutrient found in seafood and used as an antiseptic.
The common thread running through all these elements is their seven valence electrons. This unique electronic configuration dictates their chemical behavior, making halogens essential players in various chemical reactions and industrial applications. Understanding the role of valence electrons in halogens provides insights into the fundamental principles of chemistry and the fascinating world of these highly reactive elements.
Group 17: The Halogen Group and Their Seven Valence Electrons
In the realm of chemistry, there exists a remarkable group of elements known as halogens, united by their seven valence electrons. These elements reside in Group 17 of the periodic table, a group affectionately dubbed the “halogen group.”
Halogens are celebrated for their highly reactive nature, a characteristic intimately linked to their unique electronic configuration. The seven valence electrons in their outermost energy level grant them an insatiable appetite for electrons, driving them to form bonds with other elements to achieve stability.
The seven valence electrons of halogens manifest in their tendency to gain or share one electron. By acquiring or sharing this electron, they attain a stable electron configuration, mirroring the noble gas configuration of their immediate neighbors in the periodic table. This shared characteristic among halogens underscores their remarkable chemical similarities.
Reactivity Due to Seven Valence Electrons
Halogens: A Group of Highly Reactive Elements
Halogens, the members of Group 17 on the periodic table, possess a unique characteristic that sets them apart from other elements: seven valence electrons. These electrons, located in the outermost energy level of the atom, play a crucial role in determining the chemical reactivity of halogens.
The Importance of Valence Electrons
Valence electrons are the electrons that participate in chemical reactions, and their number influences an element’s ability to form bonds with other atoms. Halogens, with their seven valence electrons, are highly reactive due to their tendency to gain or share one electron to achieve a stable electron configuration.
Ionic and Covalent Bonds
When halogens react with metals, they tend to gain one electron to complete their valence shell of eight electrons, forming ionic bonds. For example, when chlorine reacts with sodium, it gains an electron from sodium to become a chloride ion (Cl-).
On the other hand, when halogens react with nonmetals, they often share valence electrons to form covalent bonds. For instance, fluorine reacts with hydrogen to form hydrogen fluoride (HF), where each atom contributes one valence electron to form a shared pair of electrons.
The Power of Seven
The reason for the high reactivity of halogens lies in their specific number of seven valence electrons. With seven electrons in their outermost shell, halogens are just one electron short of completing a stable octet of electrons. This makes them eager to react with other atoms to achieve this stable configuration.
The presence of seven valence electrons is a defining characteristic of halogens. It grants them a high level of chemical reactivity, enabling them to form ionic or covalent bonds with a wide range of elements. Understanding the significance of valence electrons helps us comprehend the unique properties and behavior of these highly reactive elements.