Guide To Periodic Table Rows: Understanding Periods And Their Significance
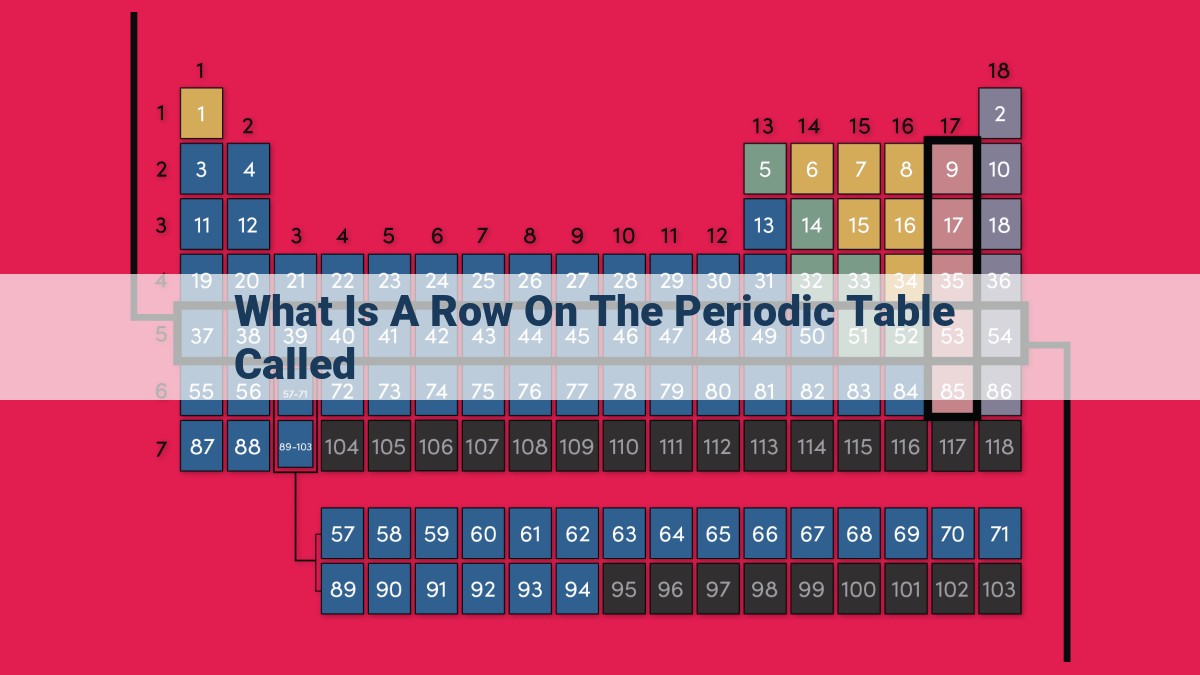
A row on the periodic table, known as a period, represents a horizontal sequence of elements sharing the same energy level for their outermost electrons. There are seven periods in total, numbered 1 to 7, indicating the number of energy levels. Rows differ from columns (groups) and diagonal lines (blocks) within the periodic table. Elements in a period share similar chemical properties due to their identical outermost energy level. Alternative terms for rows include lines, series, and vectors, all referencing their horizontal arrangement and energy level connection.
Unveiling the Secrets of the Periodic Table: Discovering the Rows (Periods)
As we embark on a captivating journey through the realm of chemistry, let’s unravel the mysteries of the periodic table. Its rows, known as periods, hold a treasure trove of knowledge about the elements that shape our world.
Imagine the periodic table as a grand tapestry, with elements meticulously arranged in horizontal rows. These rows represent energy levels, the realms where electrons reside. Each row, or period, contains elements with electrons occupying the same energy level.
The table comprises seven periods, numbered 1 to 7. Each period corresponds to the number of energy levels an element possesses. Think of these energy levels as layers of an organized cosmic dance, with elements in each layer sharing a common rhythm.
It’s important to note that rows, or periods, are distinct from columns (groups) and diagonal lines (blocks) within the periodic table. While rows represent energy levels, columns and blocks focus on other fundamental properties of elements.
Now, let’s zoom in on a specific row. Each element within that row shares the same energy level for its outermost electrons. These outermost electrons play a crucial role in determining an element’s chemical properties and behavior.
The term “period” is not the only way to describe rows on the periodic table. Chemists also use terms like “lines,” “series,” and “vectors.” These terms all emphasize the idea of a sequence of elements sharing a common energy level.
In essence, a row, or period, is a series of elements arranged in a specific sequence. This sequence follows the increasing order of atomic number, providing a predictable progression within each period. The concept of a series highlights the organized nature of the periodic table, where elements are not randomly placed but rather follow a logical order.
The Concept of “Period”: The Horizontal Sequence
Imagine the periodic table as a vast landscape, a tapestry of elements arranged in an intricate symphony of patterns and relationships. Rows, also known as periods, are one of the fundamental organizing principles of this chemical map. They represent a horizontal sequence of elements that share a crucial characteristic: the same energy level.
As you traverse a period from left to right, you’ll encounter elements with increasing atomic number. This means that each element has one more proton in its nucleus than the one before it. But here’s the key: despite their differences in atomic number, these elements all have the same number of energy levels.
Each energy level is like a shell around the atom’s nucleus. Electrons occupy these shells in a specific sequence, determined by their energy. The outermost shell is the one that most directly interacts with other atoms, influencing the element’s chemical properties.
In a period, all elements have electrons in the same outermost energy level. This shared characteristic gives them similar chemical behavior. They tend to have the same number of valence electrons, which are the electrons in the outermost shell that participate in chemical reactions. As a result, elements in a period exhibit a progressive change in their chemical properties, from the highly reactive metals on the left to the less reactive nonmetals on the right.
Understanding the concept of a period is crucial for deciphering the patterns and relationships within the periodic table. It provides a framework for predicting the chemical behavior of elements and unraveling the intricate dance they perform in the realm of chemistry.
The Seven Periods of the Periodic Table: A Journey through Energy Levels
The periodic table is an indispensable tool in chemistry, providing a wealth of information about the elements that make up our world. One of its most fundamental concepts is that of the period, a horizontal row of elements that share a common characteristic: the energy level of their outermost electrons.
There are seven periods in the periodic table, numbered 1 to 7. Each period corresponds to a specific energy level. As we move across a period from left to right, the atomic number of the elements increases, meaning that they have more protons and more electrons. However, their outermost electrons remain in the same energy level.
The first period contains only two elements, hydrogen and helium. These elements have their outermost electrons in the first energy level. The second period contains eight elements, from lithium to neon. These elements have their outermost electrons in the second energy level.
The third period contains eight elements, from sodium to argon. These elements have their outermost electrons in the third energy level. The fourth period contains eighteen elements, from potassium to krypton. These elements have their outermost electrons in the fourth energy level.
The fifth period contains eighteen elements, from rubidium to xenon. These elements have their outermost electrons in the fifth energy level. The sixth period contains thirty-two elements, from cesium to radon. These elements have their outermost electrons in the sixth energy level.
The seventh period is incomplete, containing only four elements: francium, radium, actinium, and thorium. These elements have their outermost electrons in the seventh energy level.
The concept of periods is crucial for understanding the chemical behavior of elements. Elements in the same period have similar chemical properties because they have the same number of outermost electrons. This similarity in chemical properties is why elements within the same period are grouped together vertically, forming the columns of the periodic table.
Rows vs. Columns and Blocks: The Horizontal and Vertical Divide of the Periodic Table
The periodic table is a treasure trove of chemical knowledge, organizing elements in a way that reveals their properties and relationships. Rows, columns, and blocks are three organizational units that provide different perspectives on these elements.
Rows, also called periods, are horizontal sequences that represent the energy levels of the elements. As you move from left to right across a row, elements gain protons and electrons, resulting in a gradual increase in atomic number. This horizontal arrangement highlights the fact that elements in the same row share the same number of electron shells.
Columns, also called groups, are vertical sequences that represent the number of valence electrons in an element’s outermost shell. Elements in the same column share similar chemical properties because they have the same number of valence electrons. For instance, all elements in Group 1 (alkali metals) have one valence electron, making them highly reactive.
Finally, blocks are diagonal lines that represent the type of orbital in which the element’s valence electrons reside. There are four types of blocks: s-block, p-block, d-block, and f-block. Each block corresponds to a specific orbital shape and energy level.
This multifaceted organization of the periodic table allows us to make predictions about an element’s properties based on its position. By understanding the distinctions between rows, columns, and blocks, we can unlock the secrets of chemical behavior and deepen our appreciation for the periodic table’s elegant design.
Horizontal Rows and Energy Levels: Unraveling the Patterns Within
In the intricate tapestry of the periodic table, rows, also known as periods, play a pivotal role in organizing the elements. These horizontal sequences represent an underlying order and elegance that governs the behavior of matter.
Each row embodies a specific energy level, indicating the location of the outermost electrons in the atoms of its constituents. These electrons determine the chemical properties of an element, shaping its reactivity and interactions with other substances. As we move from left to right across a row, the number of outermost electrons increases, leading to gradual changes in chemical character.
For instance, in the second row, we encounter elements like helium (He), lithium (Li), and oxygen (O). Helium, with its two electrons, exhibits the inert properties of a noble gas. Lithium, with one outermost electron, readily loses it to form stable compounds. Oxygen, with six outermost electrons, exhibits a high affinity for gaining two more electrons, making it an oxidizing agent.
The energy levels of the outermost electrons are directly related to the distance from the nucleus. As we move down columns (groups), the elements have more energy levels, leading to more complex chemical behavior. This relationship between energy levels and position within rows and columns provides a powerful tool for predicting and understanding the properties of elements.
Alternative Terms for Rows: Lines, Series, Vectors
In the realm of chemistry, the periodic table serves as an invaluable guide, organizing elements based on their properties and behaviors. Within this table, rows play a crucial role, representing the horizontal sequence of elements. But did you know that rows are not the only term used to describe these essential divisions?
Alternative terms, such as lines, series, and vectors, also capture the essence of rows, offering unique perspectives on their significance.
Lines aptly describe the linear arrangement of elements within a row. Each line corresponds to a specific energy level, indicating the location of the outermost electrons in the elements it contains.
Series emphasizes the sequential nature of rows. Elements within a series are arranged in order of increasing atomic number, the number of protons in their nuclei. This sequence reveals a gradual progression in atomic structure and properties.
Finally, the term vectors alludes to the directionality of rows. Each row represents a vector, pointing from left to right, that corresponds to the increasing number of energy levels across the table.
These alternative terms provide valuable insights into the structure and significance of rows within the periodic table. By understanding the connections between rows, lines, series, and vectors, we gain a deeper appreciation for the organization and patterns that underpin this fundamental tool in chemistry.
Rows as Series: The Sequential Nature
Unveiling the Arrangement of Elements
A row on the periodic table, often called a period, is not just a haphazard arrangement of elements. It’s a carefully orchestrated series, where each element holds its place based on an intriguing pattern – their increasing atomic number. This atomic number, like a unique identifier, represents the number of protons within the element’s nucleus.
The Story of a Row
Imagine a row on the periodic table as a fascinating tale, with each element playing a distinct role. As you move from left to right, you witness a sequential progression of elements, each with one more proton than the previous. This gradual increase in atomic number unfolds the narrative of the row, revealing the elements’ unique identities and properties.
Order and Structure
The arrangement of elements in a row isn’t arbitrary. It follows a meticulous order, determined by their atomic numbers. This order provides a framework for understanding the chemical behavior of elements and their relationships to one another.
Progressive Changes
As you traverse a row from left to right, you embark on a journey of progressive changes. The elements exhibit gradual shifts in their properties, such as atomic radius, electronegativity, and ionization energy. These changes reflect the increasing number of electrons and protons, creating a distinctive pattern within each row.
Implications for Chemistry
The sequential nature of rows has profound implications for chemistry. It allows us to predict the properties of elements based on their position within a period. By understanding the order and progression of elements, we can unravel the mysteries of chemical bonding, reactivity, and the formation of compounds.
Related Concepts: Sequence, Progression, Order
- Discuss how the concept of a series pertains to the arrangement of elements in a period, highlighting the order and progression present.
Related Concepts: Sequence, Progression, Order
The arrangement of elements within a period on the periodic table is not haphazard. Instead, it follows a logical sequence, progression, and order that provides valuable insights into the properties and behavior of these elements.
Each period, or horizontal row, represents a particular energy level. As you move from left to right across a period, the atomic number of the elements increases sequentially. This means that the number of protons and electrons in the atoms increases by one for each element.
This sequential arrangement creates a progressive change in the properties of the elements within a period. For instance, as you move from left to right, the elements generally become more metallic, less reactive, and have higher ionization energies.
The order in which the elements are arranged in a period is not arbitrary but reflects their electronic structure. The outermost electrons, which determine the chemical properties of an element, are located in the same energy level for all elements within a period. This shared energy level is the basis for the horizontal arrangement of the elements in the periodic table.
The concept of a series, or sequential arrangement, is fundamental to understanding the periodic table. The progression of elements from left to right within a period showcases the systematic changes in their properties and provides a framework for predicting the behavior of yet-undiscovered elements.