Gold Density: A Vital Property For Its Value And Applications
The density of gold, a measure of its mass per unit volume, is a crucial property that distinguishes it from other materials. Expressed in units like grams per cubic centimeter (g/cm³), it reflects the compactness of gold’s atomic structure. Various methods, such as buoyancy and weighing, are employed to accurately measure gold’s density, which is influenced by factors like temperature and purity. Gold’s high density makes it valuable in jewelry, dentistry, electronics, and coinage due to its durability and resistance to deformation. Historically, density played a significant role in establishing the gold standard and ensuring the authenticity of precious objects.
- Hook the reader with an intriguing statement about the importance of gold’s density.
- Briefly define the concept of density as mass per unit volume.
Density: A fundamental measure of the physical world, defined as the mass per unit volume. Every object, be it a feather or a nugget of gold, possesses a unique density that defines its essence. Among the tapestry of elements, gold stands out with an extraordinary density that has captivated humankind for centuries.
Gold’s high density, symbolized by its weighty feel, reflects its compact internal structure, where atoms are tightly packed, creating a substantial substance. Understanding the significance of gold’s density unlocks a realm of applications and historical implications that have shaped civilizations throughout time.
Units of Density: Unraveling the Numerical Language
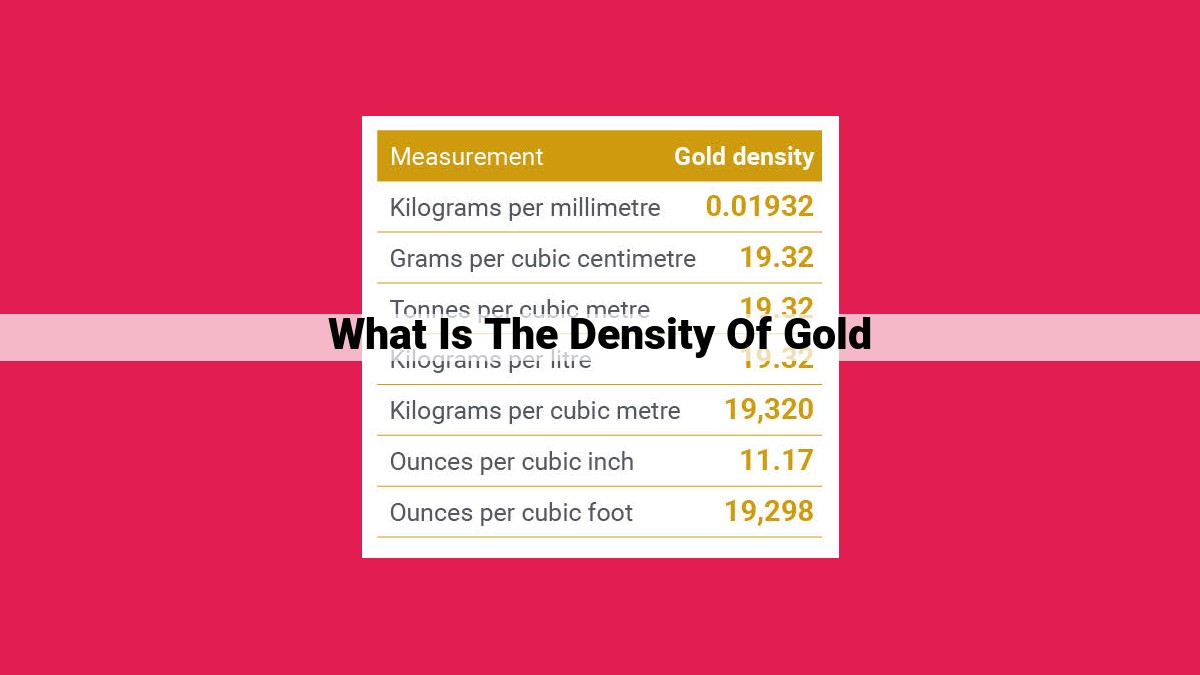
When quantifying density, we employ various units. The most common unit, grams per cubic centimeter (g/cm³), represents the mass of gold in grams contained within a cubic centimeter of volume. For larger volumes, kilograms per cubic meter (kg/m³) offers a convenient alternative. Converting between these units is a simple matter of scaling factors; 1 g/cm³ equals 1000 kg/m³.
Exploring Measurement Techniques: Unveiling Gold’s Density
Determining gold’s density involves a range of techniques. The buoyancy method, akin to Archimedes’ eureka moment, leverages the upward force exerted by a fluid to calculate density. Pycnometers, precise glass vessels, provide another means of measuring density by measuring the volume of a liquid displaced by a gold sample. Weighing scales, coupled with volume measurements, offer a straightforward approach.
Factors Influencing Density: The Dance of Temperature, Pressure, and Impurities
Gold’s density, like a symphony, responds to the interplay of external factors. Temperature, a conductor, alters the spacing between atoms, affecting density. Pressure, an external force, compresses the atomic structure, increasing density. And impurities, like guest dancers joining the gold lattice, disrupt the symmetry, influencing density.
Applications of Gold’s Density: A Versatile Substance in Modern Life
Gold’s high density endows it with remarkable properties that find applications in diverse industries. Jewelry, known for its aesthetic allure, benefits from gold’s durability and resistance to wear. Dentistry, with its focus on precision, harnesses the density of gold for sturdy dental crowns and fillings. In electronics, gold’s excellent conductivity and corrosion resistance make it a key component in connectors and circuit boards. And let us not forget the eternal allure of gold in coinage, where its density ensures durability, authenticity, and value.
Historical Significance of Gold’s Density: A Hallmark of Trust
Throughout history, gold’s density has played a pivotal role in the establishment and maintenance of the gold standard, a monetary system anchored in the intrinsic value of gold. The ability to accurately measure density has been crucial in verifying the authenticity of gold, preventing counterfeiting, and safeguarding financial integrity.
Comparison to Other Materials: A Matter of Density
Comparing gold’s density to other materials reveals its exceptional nature. Lead, with a density of 11.34 g/cm³, pales in comparison to gold’s impressive 19.3 g/cm³. Aluminum, at 2.70 g/cm³, is a lightweight contrast, highlighting gold’s substantial heft. Wood and water, both essential to life, boast densities of 0.5 g/cm³ and 1 g/cm³, respectively, further accentuating gold’s weighty presence.
Measuring the Density of Gold: A Story of Precision and Purity
Units of Density: The Scales of Measurement
When we measure the density of gold, we’re essentially determining its mass per unit volume. Density is an intrinsic property of a material, telling us how tightly packed its atoms are. In the case of gold, its high density is a hallmark of its atomic structure and purity.
The most commonly used unit of density in the scientific world is grams per cubic centimeter (g/cm³). This unit represents the mass of gold (in grams) contained within a one-cubic-centimeter volume. Another common unit, often used in larger-scale measurements, is kilograms per cubic meter (kg/m³). One kilogram per cubic meter is equivalent to 1,000 grams per cubic centimeter.
Conversion Quandary: Bridging the Units
Understanding the conversion factors between different density units is crucial for accurate data interpretation. To convert from g/cm³ to kg/m³, simply multiply the g/cm³ value by 1,000. Conversely, to convert from kg/m³ to g/cm³, divide the kg/m³ value by 1,000.
For example, if you have a gold sample with a density of 19.3 g/cm³, you can convert it to kg/m³ as follows:
19.3 g/cm³ * 1,000 = 19,300 kg/m³
This means that 1 cubic centimeter of your gold sample weighs 19.3 grams, and 1 cubic meter of the same gold weighs 19,300 kilograms.
Measuring the Density of Gold: Unveiling the Significance of Buoyancy, Pycnometers, and Weighing Scales
Gold, a precious metal renowned for its luxurious allure and monetary value, possesses a remarkable physical characteristic that sets it apart: its high density. This innate property plays a crucial role in shaping gold’s practical applications and historical significance. To accurately determine the density of gold, scientists employ various techniques, each with its own set of principles and accuracy levels.
Buoyancy: A Forceful Measurement
The principle of buoyancy, first elucidated by the brilliant Archimedes, provides a foundation for measuring gold’s density. This method involves suspending a gold sample in a fluid of known density. The buoyant force exerted on the sample, equal to the weight of the fluid displaced, is precisely calibrated to determine the sample’s volume. Combining the measured mass with the calculated volume yields the coveted density value.
Pycnometers: Precision in a Glass Vessel
Pycnometers, meticulously crafted glass containers with precisely calibrated volumes, offer another reliable method for determining gold’s density. By meticulously filling a pycnometer with a liquid of known density and then carefully introducing a gold sample, scientists can measure the change in volume. From this data, the density of the gold sample can be precisely calculated.
Weighing Scales: Simplicity and Accuracy
Weighing scales, while seemingly rudimentary, provide a straightforward and accurate means of measuring gold’s density. By meticulously weighing a gold sample in air and then submerging it in a fluid of known density, the difference in weight represents the buoyant force. This value, coupled with the sample’s mass and the fluid’s density, allows for the precise determination of the gold’s density.
Each of these techniques possesses inherent advantages and limitations. Buoyancy methods excel in measuring irregularly shaped samples, while pycnometers provide exceptional precision for smaller samples. Weighing scales offer simplicity and accuracy, making them a versatile choice for a wide range of applications.
By mastering the intricacies of these measurement techniques, scientists and artisans gain the power to accurately determine the density of gold, unlocking its full potential in a myriad of applications. From ensuring the purity of gold jewelry to verifying the authenticity of historical artifacts, the measurement of gold’s density plays a pivotal role in safeguarding the integrity of this precious metal.
Factors Affecting Gold’s Density
The density of gold, a hallmark of its intrinsic value and unique properties, is not a static quantity. This precious metal’s density is influenced by a myriad of factors, ranging from temperature and pressure to the presence of impurities. Comprehending the intricacies of these factors provides invaluable insights into the behavior and applications of gold.
Temperature’s Impact
Gold’s density exhibits an inverse relationship with temperature. As temperature rises, the atomic vibrations within the gold lattice become more pronounced, causing the metal to expand and its density to decrease. Conversely, lower temperatures result in a tighter atomic packing, increasing gold’s density. This temperature dependence is particularly crucial in high-precision applications, such as scientific instruments and electronic components, where dimensional stability is paramount.
Pressure’s Role
Pressure, like temperature, exerts a significant influence on gold’s density. As pressure increases, the gold atoms are forced closer together, resulting in a more compact structure and higher density. However, this effect is relatively minor compared to the impact of temperature and is typically only relevant in extreme pressure environments, such as those encountered during geological processes or in the depths of the Earth’s crust.
Impurities’ Influence
The presence of impurities, even in trace amounts, can significantly alter the density of gold. Impurities, such as silver and copper, disrupt the regular arrangement of gold atoms, creating voids and imperfections that reduce the overall density. The extent of this effect depends on the type and concentration of the impurities present. In applications where high purity is essential, such as financial transactions and jewelry making, meticulous measures are taken to minimize the introduction of impurities and ensure the accuracy of density measurements.
Understanding the factors that influence gold’s density is not merely academic curiosity; it holds critical implications for the practical applications of this precious metal. By tailoring the density of gold to specific requirements, engineers and artisans can optimize the performance and value of gold in diverse fields, including electronics, dentistry, and investment.
Gold’s High Density: A Treasured Property with Unparalleled Applications
Gold’s exceptional density, a testament to its innate heaviness, has propelled this precious metal to the forefront of various industries, where it plays a pivotal role in shaping functional and aesthetically pleasing creations.
In the realm of jewelry, gold’s density is a coveted attribute. Its heftiness imparts a sense of luxury and opulence, making it an ideal choice for crafting exquisite ornaments and wearable art. From intricate chains to opulent earrings, gold’s density ensures the pieces retain their structural integrity and resist deformation.
Dentistry also harnesses gold’s density to create durable and long-lasting restorations. Gold’s resistance to corrosion and wear makes it an excellent material for fillings, crowns, and bridges. Its high density ensures a tight fit, preventing bacteria and decay from penetrating the restored areas.
In the world of electronics, gold’s density plays a crucial role in ensuring the reliability and longevity of electronic components. Gold’s exceptional conductivity and resistance to oxidation make it ideal for electrical contacts, connectors, and circuit boards. Its high density enhances the durability of these components, ensuring they withstand harsh conditions and maintain optimal performance.
Coinage, an industry steeped in history, has long relied on gold’s density as a mark of authenticity and value. Gold coins have been used as a form of currency for centuries due to their incorruptibility and resistance to counterfeiting. The density of gold provides a telltale sign of its genuineness, making it difficult to replicate and ensuring the integrity of the monetary system.
Gold’s high density, a testament to its unique atomic structure, has elevated it to the status of a versatile and indispensable material. Its applications span a vast array of industries, where it adds value, enhances functionality, and embodies the enduring allure of this precious metal.
The Historical Significance of Gold’s Density: A Tale of Authenticity and Value
Gold, a precious metal renowned for its luster and durability, has played a pivotal role in human history. Its remarkable density has been instrumental in establishing its worth and safeguarding its authenticity.
In ancient times, the gold standard was adopted by many civilizations to stabilize their economies. The density of gold served as a reliable measure of its purity. Coins could be minted with a specific gold content, ensuring their value and preventing counterfeiting.
The ability to accurately measure gold’s density was crucial in verifying its authenticity. Gold’s high density made it distinct from other metals, and discrepancies in weight or volume could reveal impurities or fraudulent practices. Alchemists and goldsmiths developed techniques to measure density with precision, ensuring the integrity of gold used in currency and precious artifacts.
Over time, as trade and commerce flourished, the gold standard became less feasible. However, the density of gold remained a critical factor in assessing its quality and authenticity. Gold items, such as jewelry and bullion, were subject to rigorous testing to determine their true gold content.
Today, gold’s density continues to be a vital parameter in quality control. Advanced technologies allow for precise density measurements, enabling the detection of impurities and ensuring the purity and authenticity of gold products.
In conclusion, the historical significance of gold’s density cannot be overstated. From the gold standard to modern quality control measures, its density has played a pivotal role in safeguarding the value and authenticity of this precious metal. As a testament to its enduring importance, the density of gold remains a cornerstone of trust and confidence in the world of precious metals.
Gold’s Density: A Comparison with Other Materials
Gold, renowned for its lustrous beauty and monetary value, possesses a remarkable physical property that sets it apart from other elements – its high density. Understanding gold’s density is crucial not only for scientific exploration but also for its myriad applications. By comparing its density to that of other common materials, we gain valuable insights into its unique characteristics.
Lead: Heavier but Less Dense
Lead, a metal commonly used in batteries and ammunition, weighs more than gold. However, upon measuring their densities, we find an unexpected result. Lead’s density, approximately 11.3 grams per cubic centimeter (g/cm³), is substantially lower than that of gold, which stands at an impressive 19.3 g/cm³. This observation highlights that while lead may appear heavier, gold packs more mass into a smaller volume.
Aluminum: Lighter and Less Dense
Aluminum, a lightweight metal used in aerospace and construction, is another material often compared to gold. Aluminum’s density of 2.7 g/cm³ is noticeably lower than that of gold. This disparity in density explains why aluminum objects are much lighter than gold objects of the same size.
Wood: Buoyant and Less Dense
Wood, a versatile natural material used in construction and furniture, differs significantly from gold in terms of density. Wood’s average density ranges from 0.3 to 0.7 g/cm³, which is considerably lower than gold’s. This low density is why wood floats on water, while gold sinks.
Water: A Reference Point
Water, a ubiquitous liquid essential for life, serves as a convenient reference point for density comparison. Water’s density at room temperature is 1 g/cm³. Compared to water, gold is approximately 19 times denser, indicating its exceptional compactness.
Tabular Comparison: A Concise Summary
To provide a concise overview of the density comparisons, the following table summarizes the key information:
| Material | Density (g/cm³) |
|---|---|
| Gold | 19.3 |
| Lead | 11.3 |
| Aluminum | 2.7 |
| Wood | 0.3 – 0.7 |
| Water | 1 |
This table illustrates the significant variations in density among these materials, with gold standing out as the densest of the group.
Determining the Density of Gold: A Tale of Buoyancy and Liquid Displacement
Gold’s exceptional density has captivated humans for centuries, playing a pivotal role in its use as currency, jewelry, and more. Understanding how to determine this crucial property is essential for verifying authenticity and maintaining quality.
Archimedes’ Eureka Moment
One of the most renowned methods for measuring density is Archimedes’ principle. As the story goes, Archimedes, while pondering the problem of determining the purity of the king’s crown, stepped into a bath and noticed the water level rise. This observation sparked his realization that the crown’s volume could be calculated by submerging it in water and measuring the displaced volume.
Utilizing Archimedes’ Principle
To apply this principle to gold, we follow a similar procedure. First, the mass of the gold sample is precisely measured using a high-precision balance. Then, the sample is carefully submerged into a graduated cylinder filled with water. The volume of the displaced water is meticulously recorded, representing the volume of the submerged gold.
Density Calculation
Armed with the mass and volume of our gold sample, we can now determine its density. Simply divide the mass (in grams) by the volume (in cubic centimeters) to obtain the density in grams per cubic centimeter (g/cm³).
Liquid Displacement Method
Another reliable technique for measuring density is the liquid displacement method. This technique utilizes a liquid of known density, such as water. The gold sample is submerged in the liquid, and the volume of the displaced liquid is carefully measured.
Accuracy Considerations
Both Archimedes’ principle and the liquid displacement method provide accurate results when performed with precision. Calibrating the equipment and minimizing measurement errors are crucial for ensuring the reliability of the density measurements. These methods have proven invaluable for assessing the purity, authenticity, and consistency of gold products, making them indispensable tools in the world of goldsmithing and beyond.
Accuracy and Precision in Gold Density Measurements
In the realm of gold, density plays a paramount role in determining its purity and authenticity. Accurately measuring gold’s density is crucial for ensuring the integrity of gold products and preventing fraudulent practices.
Calibration is the cornerstone of precision density measurements. Calibrating measurement instruments, such as analytical balances and pycnometers, ensures their accuracy and reliability. This involves using reference materials with known densities to adjust the instrument’s readings.
Precision refers to the consistency of measurements. A precise instrument will yield similar results when measuring the same sample multiple times. To minimize measurement errors and enhance precision, it is essential to follow standard operating procedures and maintain a controlled laboratory environment.
Errors in density measurements can arise from various sources, including weighing inaccuracies, temperature fluctuations, and the presence of impurities. By carefully calibrating instruments, controlling environmental conditions, and using appropriate measurement techniques, these errors can be minimized.
The accuracy of density measurements is influenced by the resolution of the instrument used. High-resolution instruments provide more precise readings and reduce the uncertainty associated with the measurement. It is important to select an instrument with a resolution that is appropriate for the intended application.
Ensuring the accuracy and precision of density measurements is paramount for maintaining the integrity of gold products. By adhering to calibration protocols, minimizing measurement errors, and using appropriate instruments, the gold industry can guarantee the quality and authenticity of its offerings.
The Role of Density in Ensuring Gold’s Authenticity and Purity
Density as a Quality Control Parameter
In the realm of precious metals, *density plays a pivotal role as a quality control parameter, particularly for gold. Density, the mass of a substance per unit volume, is an essential characteristic that can reveal valuable insights about the purity, authenticity, and consistency of gold products.*
Purity Assessment
The _density_ of gold is a key indicator of its *purity. Pure gold, with a density of 19.3 grams per cubic centimeter (g/cm³), is significantly denser than most impurities or alloys. When gold is mixed with other metals, such as copper or silver, its density decreases in proportion to the amount of impurities present.*
By precisely measuring the density of a gold sample, refiners and jewelers can determine its purity level. A higher density indicates a higher gold content, while a lower density suggests the presence of impurities.
Authenticity Verification
Density is also crucial for verifying the authenticity of gold. Counterfeit gold items often contain less gold than they claim, or are even made entirely from other, less valuable materials. The density of such items will be significantly different from that of pure gold.
By comparing the density of a gold item to the known density of pure gold, experts can quickly identify fraudulent or adulterated products. This is especially important in the jewelry industry, where consumers rely on the authenticity of their gold purchases.
Consistency and Uniformity
For manufacturers and users of gold, density plays a role in ensuring _consistency_ and _uniformity_ within gold products. In the production of gold bars or coins, the density of the gold used should be consistent to meet specific weight and purity standards.
By measuring the density of each individual piece, manufacturers can ensure that they meet the required specifications and that the *quality_ of their gold products remains high.