Glycolysis: The Gateway To Cellular Respiration And Energy Production
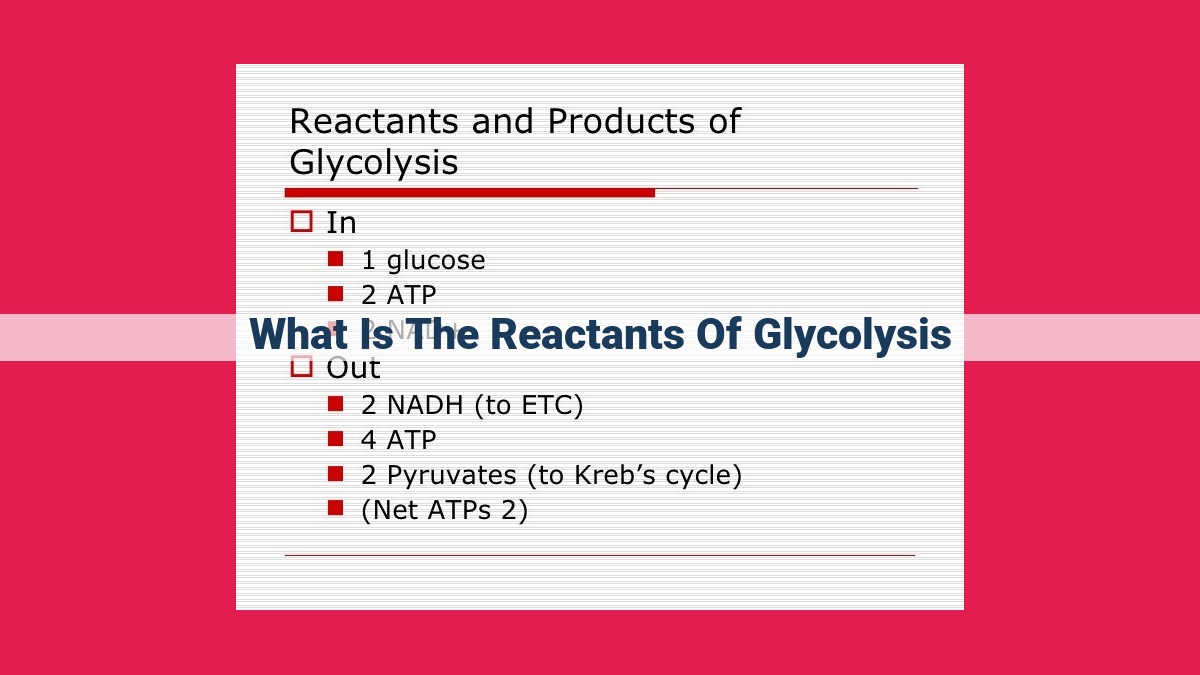
Glycolysis, the initial stage of cellular respiration, kick-starts with glucose as its primary reactant. Glucose undergoes a series of enzymatic conversions, transforming into fructose-6-phosphate and fructose-1,6-bisphosphate, the latter acting as a regulatory gatekeeper. The pathway then splits into two arms, with glyceraldehyde-3-phosphate as the branching point. Isomerization interconverts glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, ensuring metabolic balance.
Glucose: The Engine of Glycolysis
- Discuss the role of glucose as the primary reactant in glycolysis and its importance as the main energy source for most organisms.
Glucose: The Engine that Powers Life
In the realm of biology, glucose stands as the quintessential fuel that drives the metabolic machinery of most living organisms. It’s the engine that powers glycolysis, a fundamental biochemical pathway that orchestrates the breakdown of glucose to generate energy.
Glycolysis commences with glucose, the primary reactant that enters this metabolic arena. This six-carbon sugar serves as the lifeblood for countless cells, providing the energy they need to carry out their vital functions. From hummingbirds to elephants, glucose is the universal currency of cellular energy.
Fructose-6-Phosphate: A Stepping Stone in the Reaction
- Explain how glucose is converted to fructose-6-phosphate and the significance of this step as an intermediate metabolite in glycolysis.
Fructose-6-Phosphate: A Crucial Stepping Stone in Glycolysis
In the bustling metabolic city of glycolysis, glucose, the energy powerhouse, embarks on a transformative journey. It undergoes a series of chemical reactions, each carefully orchestrated to extract its valuable energy.
One of the initial steps in this intricate dance is the conversion of glucose into fructose-6-phosphate. This transformation is catalyzed by the enzyme hexokinase, which adds a phosphate group to glucose. This attachment not only locks glucose into the glycolytic pathway but also primes it for further reactions.
Fructose-6-phosphate plays a pivotal role as an intermediate metabolite in glycolysis. It’s a molecule in transition, holding valuable information about the metabolic state of the cell. Its levels fluctuate dynamically, reflecting the cell’s energy needs and availability of glucose.
Moreover, fructose-6-phosphate serves as a gateway to other metabolic pathways. It can be diverted into the pentose phosphate pathway for nucleotide synthesis or converted into fructose-1,6-bisphosphate, a key regulatory molecule that governs the rate of glycolysis.
By understanding the critical role of fructose-6-phosphate in glycolysis, we gain insights into the intricate interplay of metabolic pathways that sustain life. It’s a molecular stepping stone, paving the way for glucose’s ultimate breakdown and energy release.
Fructose-1,6-Bisphosphate: The Gatekeeper of Glycolysis
In the intricate dance of cellular respiration, glycolysis stands as the initial stage, where glucose, the body’s primary fuel, is broken down. Along this metabolic odyssey, a crucial player emerges: fructose-1,6-bisphosphate (F1,6BP). This remarkable molecule serves as a pivotal regulatory gate, governing the flow of glucose through glycolysis.
Imagine F1,6BP as a traffic cop, standing sentry at a busy intersection. It has the power to either accelerate or decelerate the rate of glucose breakdown, ensuring that cells receive a steady supply of energy.
At the heart of F1,6BP’s regulatory prowess lies its unique structure. This compound contains two phosphorylated carbons, which grant it a highly charged, reactive nature. This characteristic allows F1,6BP to interact with various enzymes involved in glycolysis, modulating their activity and thus controlling the pathway’s overall pace.
One of the most important interactions occurs between F1,6BP and 6-Phosphofructo-1-kinase (PFK-1), an enzyme that catalyzes the conversion of fructose-6-phosphate to F1,6BP. When F1,6BP levels are high, it binds to PFK-1, inhibiting its activity. This in turn slows down the production of F1,6BP and consequently, the rate of glucose breakdown.
Conversely, when F1,6BP levels are low, PFK-1 is released from inhibition. This increases the production of F1,6BP and prompts a surge in glucose breakdown.
F1,6BP’s regulatory influence extends beyond PFK-1. It also interacts with other enzymes in glycolysis, such as fructose-bisphosphate aldolase and triose-phosphate isomerase. By fine-tuning the activity of these enzymes, F1,6BP ensures that the glycolytic pathway proceeds smoothly and efficiently.
In conclusion, fructose-1,6-bisphosphate is a pivotal regulatory molecule that orchestrates the flow of glucose through glycolysis. Its unique structure and interactions with key enzymes allow it to act as a gatekeeper, precisely controlling the rate of energy production. Understanding the importance of F1,6BP provides invaluable insights into the complex mechanisms that govern cellular metabolism.
Glyceraldehyde-3-Phosphate: The Pathway’s Branching Point
As glucose embarks on its glycolytic journey, it undergoes a series of enzymatic transformations, leading it to a crucial juncture: glyceraldehyde-3-phosphate (G3P). G3P emerges as the lynchpin of glycolysis, branching out into two distinct pathways that ultimately determine the fate of glucose.
The cleavage of fructose-1,6-bisphosphate, the immediate precursor to G3P, is catalyzed by the enzyme aldolase. This enzymatic maestro dissects the six-carbon fructose-1,6-bisphosphate into two three-carbon molecules: G3P and dihydroxyacetone phosphate (DHAP).
G3P, the prima donna of glycolysis, stands at the crossroads of two divergent metabolic paths. One path leads to the continuation of glycolysis, where G3P is oxidized and phosphorylated to yield 1,3-bisphosphoglycerate (1,3-BPG). From 1,3-BPG, glucose’s odyssey continues through a series of high-energy intermediates, culminating in the ultimate production of pyruvate and ATP.
The other path branching from G3P diverges towards gluconeogenesis, the metabolic process that synthesizes glucose from non-carbohydrate precursors. In this alternative pathway, G3P is isomerized to DHAP and subsequently converted to glucose-6-phosphate, the starting point for gluconeogenesis.
Thus, G3P, the branching point in glycolysis, holds sway over the metabolic fate of glucose, orchestrating its journey towards energy production or replenishment of glucose stores.
Dihydroxyacetone Phosphate: The Glyolytic Balancing Act
In the intricate dance of glycolysis, the breakdown of glucose for energy, a pivotal transition occurs when glyceraldehyde-3-phosphate, a key glycolytic intermediate, isomerizes to form dihydroxyacetone phosphate. This transformation is more than a mere chemical shuffle; it’s a crucial balancing act that ensures the smooth flow of metabolites through the pathway.
Dihydroxyacetone phosphate serves as a versatile player in glycolysis, capable of interconverting with glyceraldehyde-3-phosphate via an isomerase enzyme. This dynamic equilibrium ensures that the cellular concentrations of these two metabolites remain in harmony, preventing imbalances that could disrupt the pathway’s progression.
The significance of this balancing act lies in the fact that both glyceraldehyde-3-phosphate and dihydroxyacetone phosphate enter the Embden-Meyerhof-Parnas pathway, a subsequent series of reactions that ultimately generates energy-rich molecules. If the interconversion between these intermediates were impaired, the supply of substrates for the Embden-Meyerhof-Parnas pathway would be compromised, slowing down glycolysis and potentially limiting cellular energy production.
Moreover, the isomerization reaction between glyceraldehyde-3-phosphate and dihydroxyacetone phosphate is reversible, allowing the pathway to adapt to cellular needs. When energy demands are high, the equilibrium shifts towards glyceraldehyde-3-phosphate, ensuring a steady supply of substrate for the Embden-Meyerhof-Parnas pathway and maximizing energy production. Conversely, when energy needs subside, the equilibrium shifts towards dihydroxyacetone phosphate, diverting metabolites to alternative pathways, such as those involved in anabolic processes and the synthesis of cellular components.
Thus, dihydroxyacetone phosphate plays a vital role in maintaining the balance of metabolites in glycolysis, ensuring the efficient generation of cellular energy and the adaptability of the pathway to fluctuating energy demands. Its isomerization with glyceraldehyde-3-phosphate is a testament to the dynamic and finely tuned nature of metabolic processes, where each step contributes to the overall symphony of life.