Gallium: Valence Electrons And Chemical Reactivity In Group 13 Elements
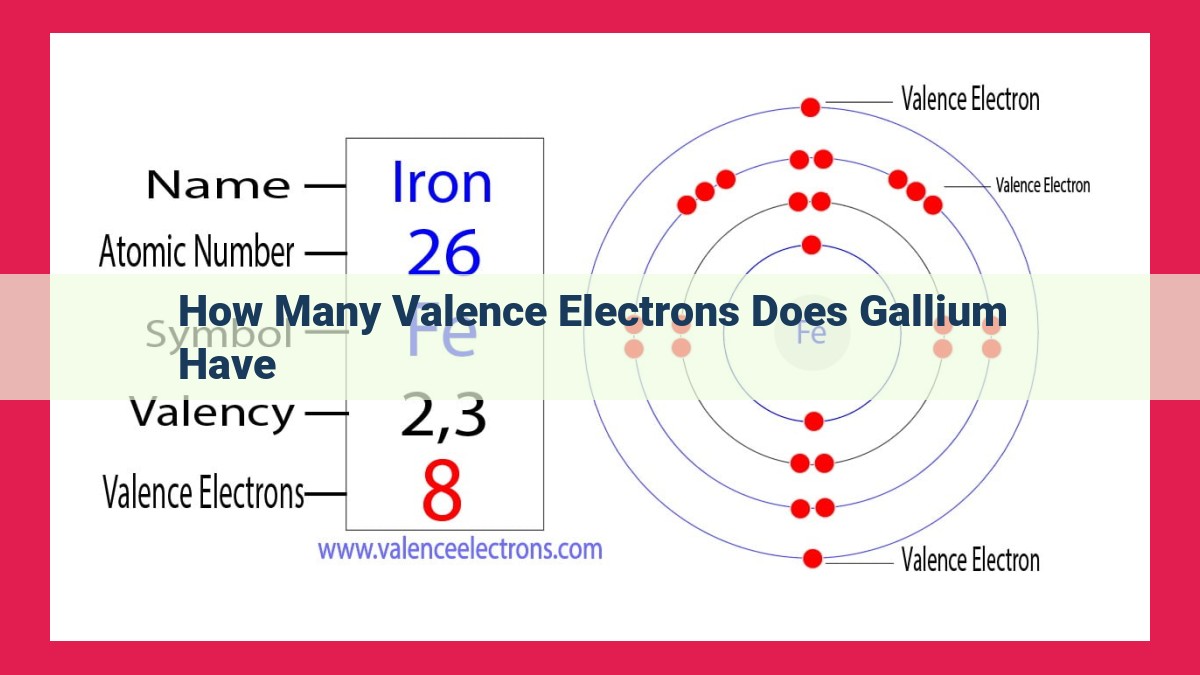
Gallium, a Group 13 element, has three valence electrons. Valence electrons are essential for determining chemical reactivity due to their presence in the outermost electron shell. Group 13 elements share this characteristic, with three valence electrons contributing to their reactivity. Gallium’s electron configuration, [Ar] 4s²3d¹⁰4p¹, reflects this, with three valence electrons in the 4p orbital. In Lewis structures, valence electrons are represented as dots or lines indicating electron pairs. Understanding the number of valence electrons in Gallium is crucial for predicting its chemical behavior and forming bonds with other atoms.
The Crucial Role of Valence Electrons: Unlocking Chemistry’s Secrets
In the vast realm of chemistry, valence electrons hold a key that unlocks the door to understanding the chemical properties of elements. These outermost electrons play a pivotal role in determining how atoms interact, leading to the rich tapestry of chemical reactions we witness around us.
Electron configuration, a fundamental concept in chemistry, refers to the arrangement of electrons within an atom’s energy levels. It provides a blueprint of an atom’s electronic structure, with valence electrons occupying the highest energy level, known as the valence shell.
Valence electrons are not mere spectators; they are the active participants in chemical reactions. Their number and arrangement dictate an element’s chemical behavior, influencing its ability to form bonds, share electrons, and participate in chemical reactions. Understanding the significance of valence electrons is essential for unravelling the intricacies of chemical processes and predicting the reactivity of different elements.
Gallium’s Place and Electron Configuration in Group 13
Imagine a group of elements, like a band of friends, with similar personalities and tendencies. This particular group, known as Group 13 or the boron group, consists of elements that share a common trait: they all have three valence electrons. Valence electrons, like the social butterflies of the atomic world, are the electrons that participate in chemical reactions, dictating an element’s chemical behavior.
Among these friendly faces, we have gallium, an element that resides in this group. Gallium, positioned neatly in the middle of the group, shares the same electron configuration as its group members. They all have their valence electrons neatly arranged in their outermost electron shell, like a trio of energetic kids playing outside.
Now, let’s compare gallium’s electron configuration with its fellow Group 13 elements. Starting with boron, the first member of the group, it has three valence electrons in its outermost shell, just like gallium. Moving downwards, we encounter aluminum, which also sports the same number of valence electrons. This pattern continues with indium and thallium, all sharing the same valence electron arrangement.
Determining Gallium’s Valence Electrons: Unraveling the Secrets of Chemical Bonding
Valence Electrons: The Key to Chemical Interactions
Imagine a social gathering where people are interacting with each other. These interactions are influenced by the number of people in the room and their respective personalities. Similarly, in the world of chemistry, valence electrons play a crucial role in determining how atoms interact with each other. These electrons, located in the outermost shell of an atom, dictate an element’s chemical properties.
Meet Gallium: A Member of the Boron Group
Gallium, an element belonging to Group 13 of the periodic table (also known as the boron group), shares a special characteristic with its groupmates: they all have three valence electrons. This unique feature has a profound impact on Gallium’s chemical behavior.
Pinpointing Gallium’s Valence Electrons
To understand how Gallium’s valence electrons influence its chemistry, let’s examine its electron configuration: 2-8-3. This configuration reveals that Gallium has two electrons in the first shell, eight electrons in the second shell, and three electrons in the outermost shell. These three valence electrons are the ones that participate in chemical reactions, forming bonds with other atoms to create compounds.
The Significance of the Valence Shell in Lewis Structures
The valence shell is the outermost electron shell of an atom, and it holds the valence electrons. Chemists use Lewis structures to represent atoms and molecules by depicting valence electrons as dots around the atomic symbol. For example, Gallium’s Lewis structure would be:
:Ga:
This structure clearly shows Gallium’s three valence electrons as dots surrounding the Ga symbol. By understanding the concept of valence electrons and their representation in Lewis structures, we gain valuable insights into the chemical behavior and properties of elements like Gallium.
The Valence Shell and Lewis Structures: Visualizing Valence Electrons
The valence shell is the outermost electron shell of an atom that contains its valence electrons. These electrons play a crucial role in determining the chemical properties of the element.
Lewis structures are a way to visualize the arrangement of valence electrons in atoms and molecules. They use dots to represent valence electrons and lines to represent chemical bonds.
By understanding the valence shell and how to draw Lewis structures, we can better comprehend the behavior of atoms and molecules. This knowledge is essential for understanding chemistry and predicting chemical reactions.
Valence electrons and Lewis structures of Gallium
Gallium is a Group 13 element, meaning it has three valence electrons. Its Lewis structure can be written as:
:Ga:
The three dots represent the three valence electrons of Gallium.
Lewis structures are important because they allow us to visualize the electron configuration of an atom or molecule and predict its chemical behavior. For example, we can use Lewis structures to predict the bonding between different atoms and the stability of molecules.