Freezing Point Of Water: Temperatures And Significance For Climate And Ecosystems
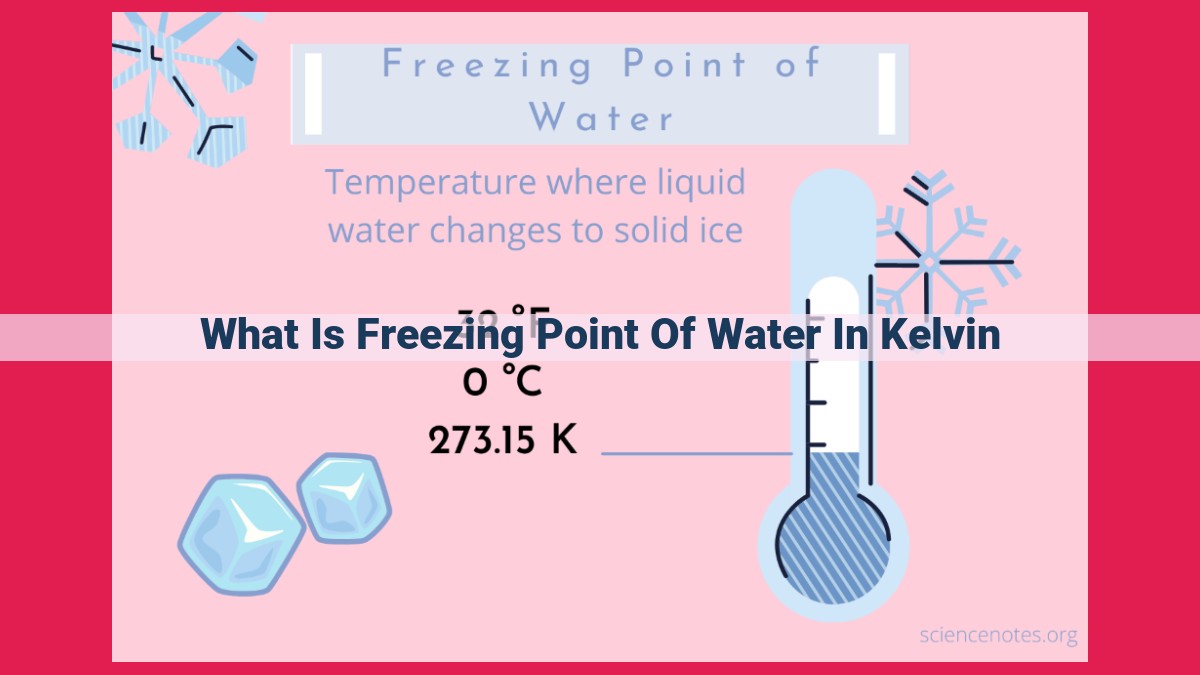
The freezing point of water is the temperature at which it transitions from a liquid to a solid state. In the Celsius scale, water freezes at 0 °C, while in the Fahrenheit scale, it freezes at 32 °F. The Kelvin scale, an absolute temperature scale, measures temperature from absolute zero (-273.15 °C). The freezing point of water in Kelvin is 273.15 K. This temperature is crucial for understanding Earth’s climate and ecosystems, as it plays a significant role in water’s phase changes and the formation of ice.
- Define freezing point and its significance in understanding temperature and phase changes.
- Introduce water as a ubiquitous substance and its importance.
Understanding the Freezing Point: A Gateway to Temperature and Phase Changes
Imagine a world where water doesn’t freeze. What would our planet and ecosystems be like? Understanding the freezing point of water is crucial for unraveling the intricate tapestry of temperature and phase changes, phenomena that shape our universe.
The Essence of the Freezing Point
The freezing point is the temperature at which a liquid turns into a solid. It marks the critical juncture where the molecular chaos of a liquid transforms into the ordered structure of a solid. This transition, driven by the delicate balance between the substance’s temperature and its internal cohesive forces, reveals profound insights into the behavior of matter.
Water: A Ubiquitous Substance
Water is the lifeblood of our planet, covering over 70% of Earth’s surface and playing a pivotal role in every ecosystem. Its unique properties, including its high freezing point, make it essential for sustaining life as we know it. Understanding the freezing point of water unveils the secrets of Earth’s climate and the intricate web of living organisms that call our planet home.
The Significance of Water’s Freezing Point
Water, the elixir of life, is an integral part of our planet’s ecosystem. Its freezing point, the temperature at which it transitions from a liquid to a solid, is a crucial factor that shapes Earth’s climate and sustains its inhabitants.
The Celsius and Fahrenheit Scales
In the realm of temperature measurement, two primary scales dominate: Celsius (°C) and Fahrenheit (°F). On the Celsius scale, water’s freezing point is conveniently designated as 0 °C, while on the Fahrenheit scale, it is designated as 32 °F. These values serve as reference points for understanding the temperature of our surroundings.
Earth’s Climate and Water’s Phase Changes
The freezing point of water plays a pivotal role in Earth’s climate. When water freezes, it releases heat into the environment, a process known as latent heat of fusion. This heat helps to regulate temperature fluctuations, particularly during seasonal changes. Additionally, the expansion of water when it freezes can cause physical changes, such as frost heaving and ice abrasion, shaping landscapes and influencing ecosystems.
Ecological Implications
The freezing point of water has profound implications for aquatic ecosystems. As water temperatures drop below freezing, many organisms, such as fish and amphibians, enter a state of dormancy known as torpor, relying on physiological adaptations to survive the cold. The availability of unfrozen water is essential for their survival, as it provides a source of oxygen and nutrients.
The freezing point of water is not merely a numerical value but a testament to the interconnectedness of our planet’s physical and biological systems. It underscores the importance of understanding temperature scales and phase changes, not only for scientific inquiry but also for appreciating the delicate balance of our environment. By delving into the significance of water’s freezing point, we gain a deeper appreciation for the intricacies of life and the need to protect our precious planet.
Kelvin: The Absolute Temperature Scale
In the realm of temperature measurement, the Kelvin scale reigns supreme as the absolute scale, providing an unwavering reference point for understanding the coldest depths of the universe.
Defined as “the fraction 1/273.16 of the thermodynamic temperature of the triple point of water,” the Kelvin scale sets its zero at absolute zero – the point where all molecular motion ceases.
At this cosmic chill, physical systems exhibit their most stable behavior, with zero energy and entropy. Absolute zero serves as a theoretical lower limit for temperature, providing a fundamental benchmark for understanding the behavior of matter and energy.
The Kelvin scale’s absolute nature eliminates the arbitrary nature of other scales, such as the Celsius and Fahrenheit scales. It provides a universal framework for temperature measurement, independent of environmental factors or the properties of specific substances.
In scientific research, the Kelvin scale is the preferred choice for expressing temperatures due to its absolute nature. It plays a pivotal role in fields such as physics, chemistry, and astronomy, where precise temperature measurements are essential for understanding the fundamental properties of matter and the behavior of the universe.
Temperature Scales: Understanding Celsius, Fahrenheit, and Kelvin
Temperature, a fundamental property of matter, is essential in comprehending the behavior of various substances. To measure temperature, scientists and everyday individuals rely on different scales—Celsius, Fahrenheit, and Kelvin—each with its unique characteristics and applications.
The Celsius scale, abbreviated as °C, is the most prevalent in the world. It is named after the Swedish astronomer Anders Celsius, who proposed the scale in 1742. On the Celsius scale, water freezes at 0 °C and boils at 100 °C at standard atmospheric pressure. This scale is widely used in everyday life, scientific research, and weather forecasting.
On the other hand, the Fahrenheit scale, abbreviated as °F, is commonly used in the United States and some Caribbean countries. Proposed by the German physicist Daniel Gabriel Fahrenheit in 1724, this scale designates the freezing point of water as 32 °F and the boiling point as 212 °F. While the Fahrenheit scale was once widely used, its prevalence has diminished in recent years.
Finally, there is the Kelvin scale, abbreviated as K, which is the absolute temperature scale. Named after Lord Kelvin, the scale was proposed in 1848 as a thermodynamically absolute scale. The Kelvin scale assigns absolute zero as its starting point, which represents the theoretical temperature at which all molecular motion ceases. Absolute zero is equivalent to -273.15 °C or -459.67 °F. This scale is crucial in scientific research and engineering applications, where precise and absolute temperature measurements are necessary.
The conversion between these temperature scales is essential in various situations. The following formulas can be used for conversions:
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
- Fahrenheit to Celsius: °C = (°F – 32) × 5/9
- Celsius to Kelvin: K = °C + 273.15
- Kelvin to Celsius: °C = K – 273.15
- Fahrenheit to Kelvin: K = (°F + 459.67) × 5/9
- Kelvin to Fahrenheit: °F = (K × 9/5) – 459.67
Understanding the different temperature scales and their interrelationships is crucial for scientists, engineers, meteorologists, and individuals in various fields. By embracing the appropriate scale for the context, we can accurately measure and interpret temperature, enabling us to better comprehend the world around us.
Celsius and the Freezing Point of Water
In the realm of temperature measurement, the Celsius scale stands prominently, its significance stemming from its profound connection to the freezing point of water. Water, a ubiquitous substance on our planet, undergoes a pivotal phase change at 0 degrees Celsius (0 °C): it transforms from a liquid to a solid state, forming ice.
This designated freezing point of water serves as a cornerstone of the Celsius scale, a testament to the scale’s precision and practicality. The freezing point of water, after all, is a universal phenomenon, easily observable and understood across diverse cultures and regions.
The Celsius scale has gained widespread prevalence in everyday life, particularly in countries outside the United States. It is used in various settings, from weather forecasts to household appliances such as ovens and thermometers. Its simplicity and intuitive nature make it a popular choice for conveying temperature information in a relatable manner.
Fahrenheit and the Freezing Point of Water
The Fahrenheit scale, named after the German physicist Daniel Gabriel Fahrenheit, is a temperature scale that assigns the value of 32 degrees Fahrenheit (°F) to the freezing point of water and 212 degrees Fahrenheit (°F) to the boiling point of water. This scale was developed in the early 18th century and was widely used in the United States until the 20th century.
Today, the Fahrenheit scale is primarily used in the United States, with most other countries adopting the Celsius scale. In scientific contexts, the Kelvin scale is the preferred scale due to its absolute nature.
The Fahrenheit scale’s designation of water’s freezing point as 32 °F is an arbitrary choice made by Fahrenheit himself. There is no scientific basis for this value, and it is simply a convention that has been followed for centuries.
The limited use of the Fahrenheit scale in scientific contexts is due to its non-linear relationship with the Kelvin scale. The Kelvin scale is an absolute temperature scale, which means that it has a true zero point at -273.15 °C, the temperature at which all molecular motion ceases. The Fahrenheit scale, on the other hand, does not have a true zero point, and its values can become negative at extremely low temperatures.
Despite its limited use in scientific contexts, the Fahrenheit scale remains a popular choice for everyday use in the United States. Most Americans are familiar with the Fahrenheit scale and use it to measure temperatures in their homes, offices, and cars. However, for scientific purposes, the Celsius or Kelvin scales are preferred due to their greater accuracy and consistency.