Freezing Point Depression And Boiling Point Elevation: Effects Of Dissolved Substances On Water
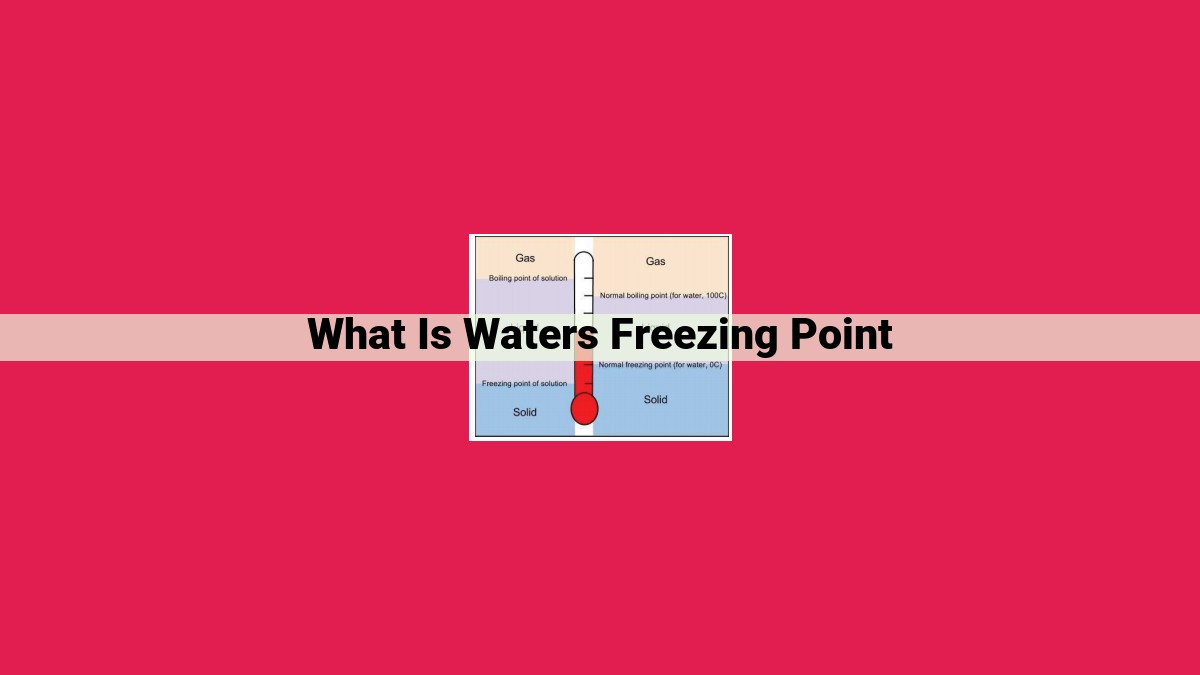
The freezing point of water is the temperature at which it transitions from a liquid to a solid phase. At sea level, the normal freezing point of pure water is 0° Celsius (32° Fahrenheit). However, dissolved substances (solutes) can alter the freezing point of water through colligative properties. Freezing point depression occurs when solutes lower the freezing point by increasing the number of particles in solution. Conversely, boiling point elevation represents the increase in boiling point caused by the presence of solutes. The Van’t Hoff factor accounts for the presence of ions in solution, which contribute additional particles and further affect freezing point depression and boiling point elevation.
Normal Freezing Point
- Explain that water’s normal freezing point is 0° Celsius (32° Fahrenheit) at sea level.
The Enigmatic World of Chemistry: Unveiling the Secrets of Freezing Point
Ice, the frozen form of water, is a ubiquitous part of our world, from the polar caps to the frosty depths of our freezers. But have you ever wondered why water freezes at 0° Celsius (32° Fahrenheit) at sea level?
The answer lies in the concept of the normal freezing point, a critical property that determines the temperature at which a pure substance like water transforms from a liquid to a solid. For water, this magical number is 0°C (32°F) under standard atmospheric pressure. Why this particular value? It’s all due to the unique molecular structure of water.
When water molecules dance freely in their liquid state, they form a network of hydrogen bonds, like tiny magnets pulling each other close. These bonds add an extra layer of stability to the liquid, making it harder for the molecules to break free and enter the more rigid, crystalline structure of ice.
But once the temperature dips below 0°C, the strength of these hydrogen bonds is overcome. Water molecules start to slow down, lose their fluidity, and line up in a regular hexagonal lattice, forming the familiar crystals of ice. This transition from liquid to solid is what we call freezing.
Colligative Properties and Freezing Point Depression
- Describe colligative properties as solution characteristics dependent on solute particle count.
- Discuss freezing point depression as the lowering of freezing point due to dissolved solutes.
Colligative Properties and Freezing Point Depression: A Tale of Dissolved Solutes
Dive into the captivating world of colligative properties, intriguing characteristics of solutions that depend solely on the number of dissolved solute particles. These properties are like the fingerprint of a solution, revealing the presence and concentration of its dissolved components.
Freezing Point Depression: The Chilling Effect of Solutes
Imagine a pristine lake, its waters frozen solid at the normal freezing point of 0° Celsius (32° Fahrenheit). But what happens when you sprinkle a handful of salt into the lake? Its freezing point magically drops, defying expectations! This phenomenon is known as freezing point depression, a direct consequence of dissolved solutes.
As solute particles dissolve in a solvent, they create a crowded environment that hinders the formation of ice crystals. This means that the water molecules have to cool down even further to overcome the disruptive effects of the solutes and freeze. The more solute particles present, the more significant the freezing point depression.
In other words, the dissolved solutes act like a team of mischievous imps, dancing around the water molecules and preventing them from settling into their frozen slumber.
Boiling Point Elevation: When Solutions Defy the Usual
While we’re accustomed to water boiling at 100°C, the presence of dissolved solutes can elevate this boiling point. This phenomenon, known as boiling point elevation, reflects the increased effort required to convert a solution into vapor compared to pure water.
The colligative properties of a solution, such as its freezing point depression and boiling point elevation, depend solely on the amount of dissolved particles rather than their specific nature. Thus, a solution with a higher concentration of solute particles will exhibit a greater boiling point elevation.
This relationship between solute concentration and boiling point elevation parallels the effect of solutes on freezing point depression. By increasing the number of solute particles, whether through direct addition or ionization, we can lower the freezing point and elevate the boiling point.
This principle finds applications in various fields, such as determining the molecular weight of substances and predicting the boiling points of solutions. For example, in cooking, adding salt to water raises its boiling point, allowing certain foods to cook faster and more evenly.
By understanding the colligative properties like boiling point elevation, we gain insight into the behavior of solutions and can harness their unique characteristics for practical applications and scientific understanding.
Exploring the Van’t Hoff Factor and Ionization: Unraveling Colligative Property Effects
Van’t Hoff Factor: A Measure of Solute Particles
In the realm of solutions, the Van’t Hoff factor is a crucial concept that measures the effective number of solute particles present. This factor is particularly significant for ionic compounds, as they dissociate into individual ions when dissolved in a solvent.
Ionization: A Game-Changer for Colligative Properties
Ionization plays a remarkable role in shaping the colligative properties of solutions. When an ionic compound dissolves, it splits into multiple ions, each acting as an independent solute particle. This significant increase in the number of solute particles has a direct impact on the freezing point depression and boiling point elevation of the solution.
Freezing Point Depression and Boiling Point Elevation: The Ripple Effect of Ionization
As the number of solute particles increases due to ionization, the effects on freezing point depression and boiling point elevation become more pronounced. The addition of ions lowers the freezing point and raises the boiling point to a greater extent compared to non-ionic compounds with the same molarity.
This enhanced effect is a direct consequence of the inflated number of solute particles caused by ionization. These particles interfere with the formation of ice crystals during freezing, depressing the freezing point. Similarly, they compete with water molecules for vaporization during boiling, elevating the boiling point.
Understanding the Implications for Solution Behavior
Comprehending the impact of the Van’t Hoff factor and ionization is essential for predicting the behavior of solutions. These concepts provide critical insights into the properties of diverse mixtures, influencing their applications in various scientific and industrial fields.
Osmosis and Semipermeable Membranes
Imagine a bustling city where tiny water molecules are frantically rushing about. These molecules are like minuscule taxis, zipping through the streets in search of their destination. However, not all streets are created equal. Some have barriers called semipermeable membranes, which allow only certain molecules to pass through.
Semipermeable membranes are like bouncers at a club, allowing only certain molecules to enter the VIP area. In this case, water molecules are the only ones invited to the party. When water encounters a semipermeable membrane, it starts knocking on the door, eager to join the fun on the other side.
But why all this commotion? It’s because the water molecules on one side of the membrane are outnumbered by solute particles. These solute particles could be anything from salt to sugar, and they’re like party crashers who are taking up all the space. To restore balance, water molecules rush to the party with the fewer solute particles, diluting the solution and creating a more harmonious environment.
This process is known as osmosis. It’s like a magic trick where water molecules defy gravity and flow against the odds, all in the name of equilibrium. Osmosis plays a crucial role in many biological processes, from keeping our cells hydrated to regulating blood pressure. Without it, life as we know it wouldn’t be possible. So next time you drink a glass of water, remember the tiny drama unfolding at the cellular level, as water molecules dance their way through semipermeable membranes, ensuring that life’s party stays balanced.
The Freezing Point Depression Constant: Unlocking the Secrets of Solvent Behavior
Have you ever wondered why adding salt to water lowers its freezing point? Or why antifreeze prevents your car engine from freezing in the winter? The answer lies in the fascinating concept of freezing point depression.
Freezing Point Depression: A Solvent’s Unique Fingerprint
Freezing point depression is the phenomenon where the presence of dissolved solute particles reduces the freezing point of a solvent. For instance, when salt (solute) is dissolved in water (solvent), the freezing point drops below 0° Celsius. This effect is caused by colligative properties, which are solution characteristics that depend solely on the number of solute particles.
The Freezing Point Depression Constant: A Solvent’s Identity Card
Each solvent has a unique freezing point depression constant (Kf), which is a numerical value that quantifies the extent of freezing point depression. This constant represents the freezing point change caused by dissolving one mole of solute in one kilogram of solvent.
Predicting Freezing Points: A Simple Equation
The freezing point depression constant allows us to accurately predict the freezing point of a solution. Using the equation:
Freezing point depression (ΔTf) = Kf x molality
where:
- ΔTf is the change in freezing point
- Kf is the freezing point depression constant for the solvent
- molality is the concentration of the solution in moles of solute per kilogram of solvent
Applications: From Road Safety to Biological Processes
Understanding freezing point depression has practical applications in various fields. In winter, antifreeze is added to car engines to prevent freezing. In the medical field, freezing point depression is used to determine the osmolality of body fluids, which is crucial for maintaining cellular function.
The freezing point depression constant is a powerful tool that provides insights into solvent behavior and enables us to predict solution properties. Its applications extend far and wide, from ensuring road safety to understanding biological processes. So, next time you add salt to your icy driveway or marvel at how antifreeze protects your car from freezing, remember the fascinating science behind it!