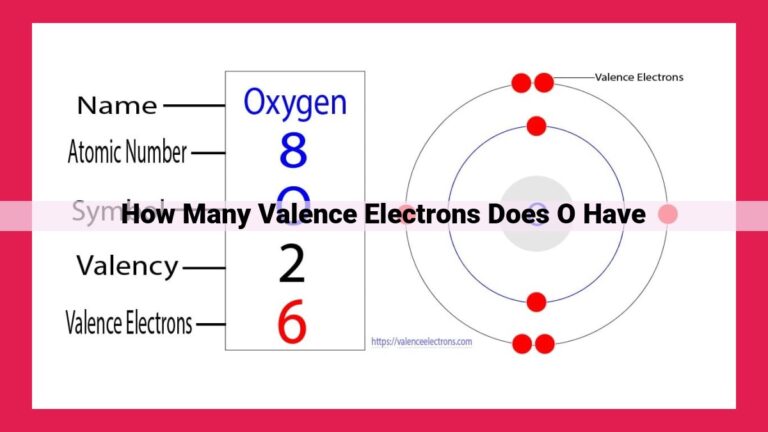
Francium: An Alkali Metal With A Unique Valence Electron
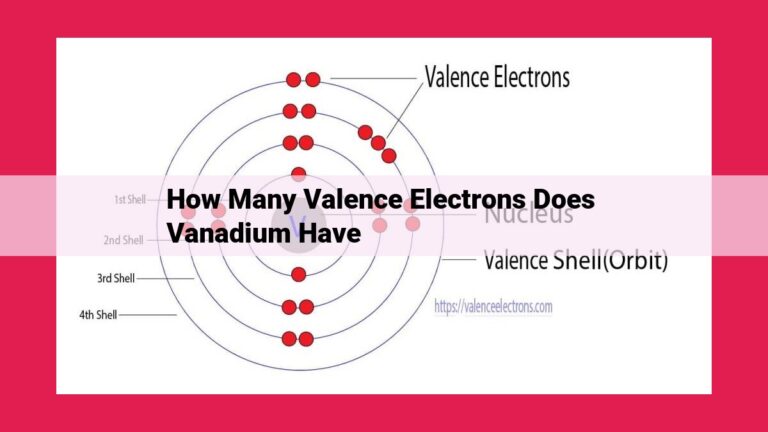
Francium, an alkali metal located in Group 1 of the periodic table, possesses a single valence electron. This valence electron plays a crucial role in determining the chemical behavior of francium, facilitating its formation of chemical bonds and interactions with other elements.
Valence Electrons: A Foundation
- Definition and characteristics of valence electrons
- Significance in chemical bonding and Lewis structures
- Correlation to atomic orbitals
Valence Electrons: The Building Blocks of Chemistry
Imagine a puzzle where each piece is a tiny atom. Valence electrons are the glue that holds these atoms together, forming the molecules that make up our world. They are the outermost electrons in an atom, eager to interact and shape its chemical destiny.
Valence electrons dance around the nucleus in specific spaces called atomic orbitals. These orbitals form the outermost shell of the atom, determining its chemical character. They are like a neighborhood surrounding the nucleus, where electrons can interact with each other and with electrons from other atoms.
The number of valence electrons an atom has is crucial for chemical bonding. In Lewis structures, which depict how atoms connect with each other, valence electrons are represented as dots surrounding the atomic symbol. By understanding the valence electrons of an atom, chemists can predict how it will bond and form new molecules.
Francium: The Radioactive Alkali
- Introduction and classification as an alkali metal
- Position on the periodic table
- Notable chemical and physical properties
Francium: The Enigmatic Radioactive Alkali
Nestled amidst the periodic table’s vibrant tapestry, francium emerges as an element shrouded in both intrigue and danger. An alkali metal, it belongs to an illustrious family renowned for its tempestuous reactivity and fascinating properties.
Francium’s atomic number of 87 places it far down the table, in the lower reaches of the Group 1 elements. Its position on the periodic table reveals its remarkable chemical and physical characteristics. Francium is the heaviest alkali metal, boasting an atomic mass of 223. This heftiness endows it with an unusually low melting point and a volatile nature. In its pure form, francium is a silvery-white metal that melts at a mere 27°C.
As an alkali metal, francium exhibits the characteristic high reactivity of its brethren. It reacts violently with water, releasing hydrogen gas and forming a caustic hydroxide. Its extreme reactivity precludes the existence of pure francium in nature, as it instantly reacts with air and water. Francium’s radioactive nature further complicates its existence. With a half-life of just 22 minutes, francium is one of the most radioactive elements known. Its atoms undergo a rapid series of decay processes, releasing alpha and beta particles and ultimately transforming into stable isotopes of radon.
Despite its fleeting existence, francium has captivated the scientific community. Its radioactive emissions have found practical applications in nuclear chemistry and medicine. Francium-223, the isotope with the longest half-life, is used as a tracer to study biological processes. Its unique properties make francium an indispensable tool in the field of nuclear medicine.
Although rare and elusive, francium remains a testament to the wonders of the chemical world. Its reactivity, radioactivity, and unique properties have solidified its place as an element of immense scientific value, offering insights into the nature of matter and the complexities of the periodic table.
The Enigmatic World of Radioactivity and Nuclear Chemistry
Unveiling the Secrets of Atomic Instability
In the depths of the atom, where invisible forces orchestrate the dance of subatomic particles, lies the fascinating realm of radioactivity and nuclear chemistry. Radioactivity, the spontaneous emission of energy and particles from an atom’s nucleus, is a testament to the unstable nature of certain elements. Francium, an elusive alkali metal, stands as a prime example of this nuclear instability.
Francium’s Radioactive Demise
Francium, with its solitary valence electron, is a highly reactive metal that readily forms chemical bonds. However, this same reactivity extends to its nucleus, making it radioactive. The imbalance within the nucleus leads to a constant struggle for stability, resulting in the emission of alpha particles (helium nuclei) and gamma rays. This radioactive decay is a natural process by which Francium seeks to attain a more stable configuration.
Harnessing Radioactivity for Progress
Despite the inherent danger associated with radioactivity, it has also proven to be an invaluable tool in the field of nuclear chemistry. The controlled manipulation of nuclear reactions has led to groundbreaking advancements in diverse areas. One prominent application is nuclear medicine, where radioactive isotopes are used to diagnose and treat medical conditions. Radioisotopes can act as tracers to illuminate metabolic processes or as therapeutic agents to target diseased tissues.
Nuclear Power and Beyond
The most well-known application of nuclear chemistry is undoubtedly nuclear power. Nuclear power plants harness the energy released by nuclear fission, the splitting of heavy atomic nuclei, to generate electricity. While this technology has provided a significant source of clean energy, it also raises important safety concerns. Additionally, nuclear chemistry plays a crucial role in nuclear waste management, ensuring the safe handling and disposal of radioactive materials.
As we continue to probe the depths of matter, the mysteries of radioactivity and nuclear chemistry will undoubtedly yield further insights and technological advancements. From understanding the fundamental nature of the atom to harnessing its power for scientific progress, this enigmatic field holds the potential to shape our future in profound ways.
Periodic Trends: Exploring the Spectrum of Atomic Properties
Across the expanse of the periodic table, a remarkable tapestry of atomic properties unfurls, reflecting the intricate interplay of electrons and atomic structure. Three fundamental trends, interconnected like threads in a celestial dance, reveal the subtle nuances that define each element: atomic radius, ionization energy, and electronegativity.
Atomic Radius: A Symphony of Size
Like planets orbiting a star, electrons occupy concentric shells around the atomic nucleus. The size of these shells, known as the atomic radius, diminishes from left to right across a period on the table. Moving downward within a group, the radius inflates due to the addition of new electron shells. This variation in size profoundly influences an element’s chemical reactivity and physical properties.
Ionization Energy: The Unwavering Guardian
Ionization energy measures the unyielding resolve with which an atom holds onto its electrons. It represents the energy required to strip an electron from its atomic embrace. As we traverse the periodic table, ionization energy generally increases from left to right and decreases from top to bottom. This trend reflects the tightening grip of the nucleus on its electrons and the increasing ease of removing them.
Electronegativity: The Allure of Attraction
Electronegativity quantifies the ability of an atom to attract electrons towards itself. It serves as a measure of the atom’s greed for electrons and has a profound impact on chemical bonding. Electronegativity increases from left to right across a period and decreases from top to bottom within a group. This gradient determines the polarity of chemical bonds and the formation of molecules.
By understanding these periodic trends, scientists unravel the secrets of the elements, predicting their behavior and harnessing their unique properties for countless applications. From the design of materials to the synthesis of novel drugs, these trends guide our exploration of the atomic realm.
Unraveling the Secrets of Francium’s Valence Electrons
In the realm of chemistry, valence electrons take center stage, holding the key to understanding the behavior and properties of elements. Francium, an elusive and radioactive alkali metal, stands out as a captivating case study. This blog post embarks on a journey to demystify the valence electrons of francium, revealing their profound influence on its chemical characteristics.
Identifying Valence Electrons
The quest to determine valence electrons begins with the periodic table, a treasure trove of elemental secrets. Francium resides in Group 1, also known as the alkali metals. This esteemed position grants francium a single valence electron, destined to play a pivotal role in its chemical dance.
Francium: An Alkali Metal with a Lone Ranger
As an alkali metal, francium embodies the essence of reactivity. Its solitary valence electron yearns to break free, embarking on a quest to bond with neighboring atoms. This singular valence electron empowers francium with an intense hunger for electrons, rendering it an eager participant in chemical reactions.
Valence Electrons: Guiding Francium’s Chemistry
Francium’s lone valence electron serves as the maestro of its chemical behavior. It orchestrates the formation of ionic bonds with non-metals, willingly surrendering this electron to achieve a stable, noble gas configuration. The presence of this single valence electron is the driving force behind francium’s high reactivity, making it an ideal candidate for use in specialized applications, such as ionization detectors.
In conclusion, the valence electrons of francium hold the key to its chemical identity. They govern its reactivity, dictate its bonding patterns, and influence its overall behavior. Understanding these fundamental particles unlocks the mysteries of this fascinating radioactive element, allowing us to appreciate its unique contributions to the world of chemistry.