Unlocking Fluorine’s Chemistry: Valence Electrons, Reactivity, And Chemical Properties
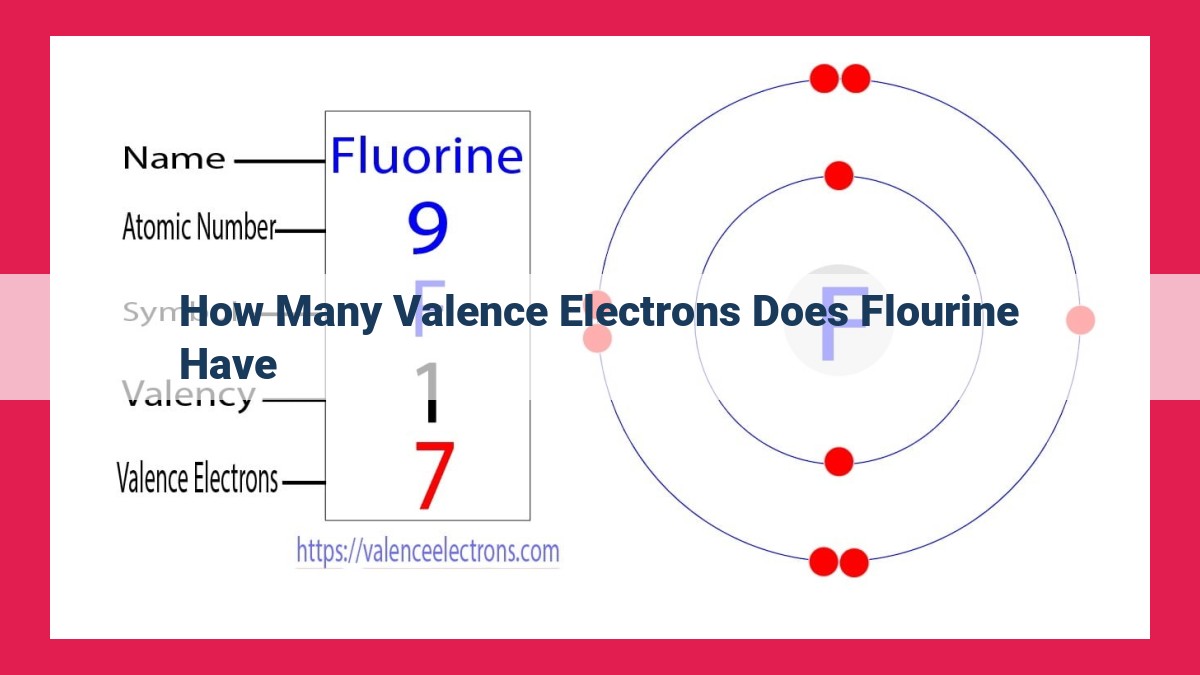
Fluorine, a halogen element, has 7 valence electrons. These valence electrons reside in its outermost 2p orbitals. Due to their high number of valence electrons, halogens like fluorine are highly reactive and tend to gain one electron to achieve a stable octet configuration. This high electronegativity and reactivity contribute to fluorine’s distinctive chemical properties and explain its role in forming various chemical compounds.
Understanding the Significance of Valence Electrons in Fluorine
The world of chemistry revolves around the intricate dance of electrons, especially when it comes to understanding the behavior of elements. Valence electrons are the key players in this dance, influencing the chemical bonding and reactivity of elements. In this blog, we’ll embark on a journey to unravel the fascinating world of valence electrons and their crucial role in shaping the properties of fluorine.
Valence Electrons: The Building Blocks of Chemistry
Valence electrons are the electrons that reside in the outermost energy level of an atom. They play a vital role in chemical bonding, as they determine an element’s ability to gain, lose, or share electrons with other atoms. This, in turn, influences the element’s chemical reactivity and the formation of compounds.
Fluorine: A Halogen with a Unique Identity
Fluorine is a fascinating element that belongs to the halogen group. Halogens are a family of highly reactive nonmetallic elements that share a common characteristic: they all have seven valence electrons. Fluorine stands out as the most electronegative element of all, meaning it has a strong tendency to attract electrons from other atoms.
Fluorine’s Valence Electrons: A Path to Understanding Its Properties
Fluorine’s unique chemical properties can be attributed to its valence electrons. With seven valence electrons, fluorine has an electron configuration of 1s² 2s² 2p⁵. This means that its outermost energy level contains five valence electrons, which are located in the 2p orbitals.
The presence of seven valence electrons makes fluorine highly reactive. It readily gains an electron to achieve a stable octet configuration (eight valence electrons), resulting in the formation of negative fluoride ions (F⁻). This high reactivity is responsible for fluorine’s tendency to form compounds with a wide range of elements.
Halogens and the Power of Valence Electrons
Fluorine is not alone in its valence electron configuration. All halogens, including chlorine, bromine, and iodine, possess seven valence electrons. This shared characteristic contributes to their similar chemical behaviors, such as their high reactivity and tendency to form negative ions.
Valence electrons are the driving force behind the chemical bonding and reactivity of elements. In the case of fluorine, its seven valence electrons play a pivotal role in shaping its properties. As the most electronegative element, fluorine exhibits a strong tendency to gain electrons and readily forms compounds with other elements. Understanding the importance of valence electrons is crucial for comprehending the behavior and applications of fluorine and other elements in the chemical world.
Valence Electrons in Fluorine: A Chemical Bonding Odyssey
In the realm of chemistry, valence electrons play a pivotal role in orchestrating the dance of atoms to form the building blocks of our world. These electrons reside in the outermost shell of an atom, determining its chemical reactivity and the bonds it can forge with others.
Fluorine, an enigmatic element from the halogen family, boasts seven valence electrons. This captivating element is highly reactive, eager to embrace an additional electron to complete its octet configuration, a stable arrangement of eight electrons in its outermost shell.
The electron configuration of fluorine elucidates the blueprint of its atomic structure: 1s² 2s² 2p⁵. This intricate notation discloses that fluorine possesses two core electrons nestled in the innermost 1s orbital and four valence electrons residing in the second energy level. Of these, three occupy the 2s orbital, and five dance gracefully in the 2p orbital.
The 2p orbital, a trio of dumbbell-shaped electron clouds, harbors the valence electrons of fluorine. These electrons are energetically poised to engage in chemical bonding, the harmonious process of atom-to-atom interactions.
Fluorine’s abundance of valence electrons bestows upon it an exceptional eagerness to form chemical bonds. This high level of reactivity renders fluorine a formidable participant in a myriad of chemical reactions. Its electronegativity, a measure of its ability to attract electrons, further enhances its bonding prowess.
In summary, fluorine’s seven valence electrons, strategically positioned in its outermost atomic orbitals, empower it to engage in chemical bonding. This inherent reactivity fuels its exceptional chemical properties, making fluorine a captivating element in the scientific tapestry of our universe.
Chemical Bonding and Valence Electrons
The valence electrons of fluorine play a pivotal role in its chemical bonding and reactivity. These outermost electrons determine how fluorine interacts with other elements to form molecules and compounds.
Fluorine possesses a remarkable seven valence electrons, residing in its 2p orbitals. This high number of valence electrons makes fluorine highly reactive. To achieve a stable configuration, fluorine seeks to gain one electron, completing its outermost electron shell and forming a stable octet of eight electrons.
This electron-gaining tendency drives fluorine’s behavior in chemical reactions. It readily combines with metals, forming positively charged ionic compounds. For instance, when fluorine reacts with sodium, it accepts an electron from the metal to form the ionic compound sodium fluoride (NaF).
Moreover, fluorine’s valence electrons facilitate the formation of covalent bonds with nonmetals, such as hydrogen and chlorine. In these bonds, fluorine shares its valence electrons with other atoms to achieve a stable electron configuration.
Halogens and Valence Electrons
Unveiling the Extraordinary Reactivity of Fluorine and Its Halogen Family
In the world of chemistry, valence electrons are like the social butterflies of atoms, determining their chemical interactions and shaping their unique properties. One element that stands out as a master of valence electron manipulation is fluorine, a highly reactive element that belongs to the halogen family. Dive into the fascinating world of fluorine and its halogen counterparts to unravel the secrets of their extraordinary reactivity.
Fluorine’s Valence Electron Dance
Fluorine boasts seven valence electrons, the highest among all halogens. These outermost electrons, residing in the 2p orbital, are eager to participate in the chemical bonding game. They give fluorine an electronegative personality, making it highly attractive to positively charged ions.
The Halogen Family: Unity in Diversity
Fluorine is not alone in its valence electron adventures. All halogens share a common trait: seven valence electrons. This commonality translates into similar chemical behaviors, including their high reactivity, especially with metals to form ionic compounds.
Electronegativity: Fluorine’s Secret Weapon
Electronegativity is a measure of an atom’s ability to attract electrons towards itself. Fluorine, with its high electronegativity, is a magnet for electrons. This explains its tendency to gain an electron and achieve a stable octet configuration, resulting in the formation of fluoride ions.
The Halogens’ Chemical Dance
The high reactivity of halogens can be attributed to their seven valence electrons, which allows them to form strong bonds with other elements. Fluorine, chlorine, bromine, iodine, and astatine, all members of the halogen family, readily react with metals to form ionic compounds, with fluorine being the most reactive due to its high electronegativity.
In conclusion, fluorine’s seven valence electrons play a pivotal role in its chemical behavior, contributing to its high reactivity and electronegativity. Understanding the significance of valence electrons in fluorine and its halogen family provides a deeper appreciation for their unique chemical properties and reactivity patterns.