Finding Pka On A Titration Curve: A Step-By-Step Guide For Students And Researchers
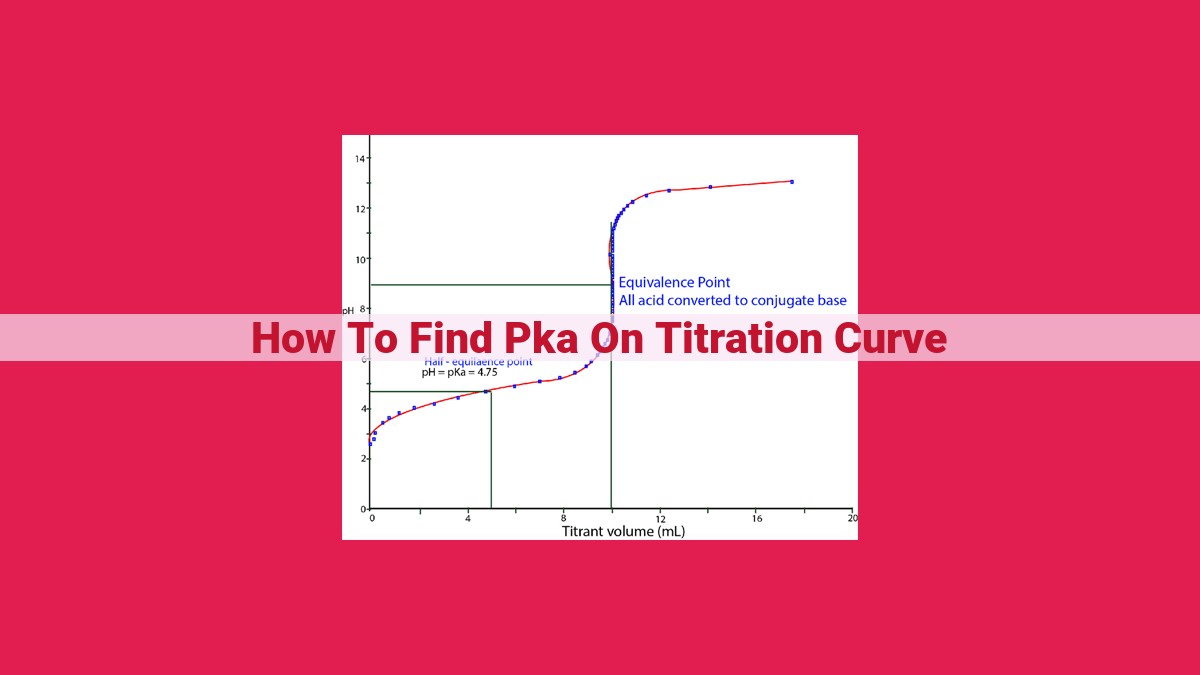
To find pKa on a titration curve: 1) Understand the basics of titration, pH, and pKa. 2) Construct a titration curve and locate the half-equivalence point, which is related to pKa. 3) Measure the pH at the half-equivalence point. 4) Calculate pKa by subtracting 1 from the pH at the half-equivalence point.
Understanding the Basics of Acid-Base Titration
Titration is a fundamental technique in chemistry that allows us to determine the concentration of an unknown acid or base. It involves carefully adding a known amount of a strong acid or base (titrant) to a sample of the unknown solution (analyte) until a specific point (equivalence point) is reached.
At the equivalence point, the moles of titrant added are equal to the moles of analyte present, and the solution has been neutralized. This point can be detected by a variety of means, such as a color change or pH measurement. The pH of a solution is a measure of its acidity or alkalinity, and it plays a crucial role in titration. Acid-base reactions involve the transfer of protons (H+) between molecules, and the pH of a solution indicates the concentration of H+ ions present.
Weak acids are acids that only partially dissociate in water. This means that they exist in equilibrium with their conjugate bases, and their dissociation constant (pKa) is a measure of their strength. The Henderson-Hasselbalch equation is a mathematical equation that relates the pH of a solution containing a weak acid to its pKa and the concentrations of the acid and its conjugate base.
Titration Curves and the Half-Equivalence Point
As we delve deeper into the world of titrations, it’s essential to understand the intricate relationship between titration curves and the enigmatic half-equivalence point. These concepts hold the key to unlocking the mysteries of acid-base reactions and deciphering the secrets of chemical solutions.
Titration Curves: A Tale of pH Ups and Downs
A titration curve is a graphical representation that charts the pH of a solution as a titrant (a solution of known concentration) is gradually added to the analyte (a solution of unknown concentration). As the titrant is added, the pH of the analyte undergoes a gradual transformation, revealing the underlying nature of the acid-base system at play.
The Significance of the Half-Equivalence Point
Within the realm of titration curves lies a pivotal point known as the half-equivalence point. This point represents the moment when half of the analyte has reacted with the titrant. It’s akin to reaching the midway point on a journey, marking a significant milestone in the titration process.
Half-Equivalence Point and pKa: Unveiling the Secrets of Acids
The pKa (dissociation constant) is a numerical measure of the strength of an acid. It reflects the acid’s tendency to donate protons (H+ ions). Notably, there’s a profound connection between the half-equivalence point and pKa that holds the key to unraveling the secrets of acid dissociation.
By investigating the titration curve, we can pinpoint the pH at the half-equivalence point. This pivotal piece of information, when combined with the Henderson-Hasselbalch equation, enables us to calculate the pKa. It’s a crucial step in identifying the strength of the acid under scrutiny.
Finding pKa on a Titration Curve
Determining the dissociation constant (pKa) of a weak acid is crucial in understanding its behavior in solution. Titration curves provide a graphical representation of the pH change as a strong base is gradually added to an acid solution. By analyzing these curves, we can conveniently calculate pKa.
Identifying Equivalence and Half-Equivalence Points
The equivalence point marks the complete neutralization of the acid, where the moles of added base equal the moles of acid. On the titration curve, it corresponds to the steepest part of the curve. The half-equivalence point occurs when half of the acid has been neutralized. It appears as an inflection point on the curve, where the slope changes abruptly.
Measuring pH at the Half-Equivalence Point
Accurately determining the pH at the half-equivalence point is essential for calculating pKa. Use a pH meter or color-changing indicator to measure the pH at this point. Note the pH value, which is usually close to the pKa of the acid.
Calculating pKa from Half-Equivalence Point
The Henderson-Hasselbalch equation provides a simple formula for pKa calculation:
pKa = pH - 1
Plug in the pH value measured at the half-equivalence point into the formula to calculate pKa. Subtracting 1 from the pH accounts for the dissociation of the weak acid and its conjugate base at the half-equivalence point.
Example:
Let’s find the pKa of an unknown weak acid. A titration experiment yields the following data:
- Equivalence point: pH = 8.0
- Half-equivalence point: pH = 6.5
Using the Henderson-Hasselbalch equation:
pKa = 6.5 - 1
pKa = 5.5
Therefore, the pKa of the unknown weak acid is 5.5.