Finding And Converting Chemical Moles: A Guide To Molar Mass, Stoichiometry, And Dimensional Analysis
To find the mole of a compound, determine its molar mass from its molecular formula. Using the formula moles = mass (g) / molar mass (g/mol), calculate the moles of the compound using its mass. Stoichiometry helps determine the moles of reactants or products involved in a chemical reaction. Dimensional analysis allows conversion between moles and grams using the molar mass as a conversion factor. Accurate mole calculations are crucial for stoichiometric calculations and conversions in chemical reactions.
Understanding the Mole Concept
A mole is a fundamental unit in chemistry that describes the number of particles present in a given quantity of a substance. In essence, it’s a convenient way to quantify a vast number of molecules, atoms, or ions.
Just like we count objects using units like dozen or hundreds, a mole allows us to count incredibly small particles in a way that makes chemical calculations manageable. The mole concept is essential because it provides a bridge between the macroscopic world we see and the microscopic world of atoms and molecules.
Knowing the number of particles involved in a chemical reaction is crucial for understanding how substances interact with each other. The mole concept provides a common ground for comparing and manipulating quantities of different substances, enabling accurate predictions of reaction outcomes and quantitative analysis of chemical processes.
Calculating Moles from Mass: Unveiling the Secrets of Chemical Proportions
In the realm of chemistry, understanding the concept of the mole is paramount. It allows us to quantify the number of particles involved in reactions and accurately predict the outcome of chemical processes. One crucial aspect of mole calculations is determining the moles of a substance from its given mass.
The Formula: A Gateway to Mole Determination
The foundational formula for calculating moles from mass is:
moles = mass (g) / molar mass (g/mol)
Here, moles represent the number of moles present, mass is the given mass of the substance in grams, and molar mass is a substance-specific constant that indicates the mass of one mole of that substance.
Molar Mass: The Key to Unlocking the Moles
Molar mass is the bridge between mass and moles. It represents the mass (in grams) of one mole of a substance. Knowing the molar mass is essential for accurate mole calculations.
Determining molar mass involves understanding the molecular formula of the substance. Each element in the formula contributes to the overall molar mass based on its atomic mass. By adding the atomic masses of all the elements present, we arrive at the molar mass of the substance.
A Step-by-Step Guide to Mole Calculations
- Obtain the mass of the substance: Determine the mass of the substance given in grams.
- Find the molar mass: Calculate the molar mass of the substance using the molecular formula and atomic masses.
- Apply the formula: Use the formula moles = mass (g) / molar mass (g/mol) to calculate the number of moles present.
Precision in Mole Calculations: The Cornerstone of Stoichiometry
Accurate mole calculations are vital in stoichiometry, a branch of chemistry that involves understanding and predicting the quantities of reactants and products involved in chemical reactions. By precisely calculating the moles of reactants and products, we can determine the limiting reactant and predict the amount of product formed.
Mastering mole calculations from mass is a cornerstone of chemistry. It empowers us to quantify the number of particles involved in reactions, accurately predict outcomes, and delve deeper into the intricate world of chemical proportions.
Understanding Molar Mass: The Foundation of Chemistry
Definition
In the realm of chemistry, the mole concept holds immense significance. A mole represents a colossal number of particles, precisely 6.022 x 10^23. Understanding the mole concept and determining molar mass are indispensable skills for unraveling the mysteries of chemical reactions.
The Significance of Molar Mass
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It serves as a fundamental property that allows us to interconvert between the mass and the number of particles in a substance.
Calculating Molar Mass from Molecular Formula
Calculating the molar mass of a compound is a straightforward process. To embark on this journey, we begin with the molecular formula of the compound. This formula provides us with the types and the number of atoms present in each molecule.
For each element in the molecular formula, we look up its atomic mass from the periodic table. Atomic mass represents the mass of one atom of an element, measured in atomic mass units (amu).
To determine the molar mass, we simply add up the atomic masses of all the atoms present in the molecular formula. The resulting value is expressed in grams per mole.
Example:
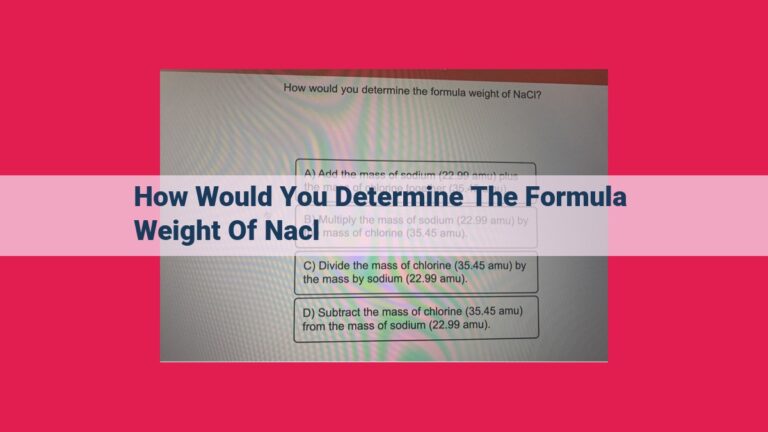
Let’s determine the molar mass of sodium chloride (NaCl).
- Sodium (Na) has an atomic mass of 22.99 amu.
- Chlorine (Cl) has an atomic mass of 35.45 amu.
Molar mass of NaCl = (22.99 amu + 35.45 amu) = 58.44 g/mol
Calculating Moles of a Compound: A Guide through Stoichiometry
In chemistry, balancing chemical equations is crucial, and the mole concept is fundamental to this process. Let’s delve into how to calculate the moles of a compound using stoichiometry and the mole ratio determined from a balanced equation.
Understanding Stoichiometry and Mole Ratios
Stoichiometry involves understanding the quantitative relationships between reactants and products in a chemical reaction. A balanced chemical equation provides the mole ratio, which indicates the number of moles of reactants and products involved in a particular reaction.
Step-by-Step Guide to Calculating Moles of a Compound
Step 1: Balance the Chemical Equation
Ensure that the equation is balanced, meaning the number of atoms of each element is equal on both sides.
Step 2: Identify the Mole Ratio
For a reaction like (aA+bB \rightarrow cC+dD), the mole ratio of A to C is (a:c). This means for every (a) moles of A, (c) moles of C are produced.
Step 3: Determine the Known Value
Find the given quantity, either mass or moles, of one of the reactants or products.
Step 4: Convert to Moles
If the given value is in mass, convert it to moles using the molar mass of the substance. Moles = Mass (g) ÷ Molar Mass (g/mol)
Step 5: Use the Mole Ratio
Using the mole ratio determined from the balanced equation, calculate the moles of the desired compound. For example, if you know the moles of A and need to find the moles of C, use the ratio (a:c).
Step 6: Check Your Answer
Verify that the mole ratio is consistent throughout the calculation.
Example
Calculate the moles of sodium chloride (NaCl) produced when 10.0 g of sodium (Na) reacts completely with excess chlorine (Cl2).
Step 1: Balanced equation: (2Na+Cl2 \rightarrow 2NaCl)
Step 2: Mole ratio: Na to NaCl is 2:2
Step 3: Given value: 10.0 g of Na
Step 4: Moles of Na: 10.0 g ÷ 22.99 g/mol = 0.435 mol
Step 5: Moles of NaCl: 0.435 mol Na × (2 mol NaCl / 2 mol Na) = 0.435 mol NaCl
Calculating moles of a compound is essential for stoichiometric calculations and conversions. By understanding the mole ratio in balanced equations, you can accurately determine the amount of a substance involved in a chemical reaction.
Converting between Moles and Grams: Unlocking Precise Measurement in Chemistry
In the vast world of chemistry, knowing the exact number of particles involved in a reaction is paramount. Enter the concept of moles, a unit that allows us to quantify these minuscule particles with astonishing precision.
When calculating moles, we often encounter the need to convert between mass and moles. This is where we encounter one of the most powerful tools in chemistry: dimensional analysis. This technique enables us to leverage known values and relationships to determine unknown quantities.
At the heart of dimensional analysis lies the conversion factor. In the context of mole calculations, this conversion factor is the molar mass. Molar mass, measured in grams per mole (g/mol), represents the mass of one mole of a substance. By utilizing molar mass as a conversion factor, we can effortlessly switch between moles and grams.
Let’s take an example. Suppose you want to determine the number of grams of sodium chloride (NaCl) in 0.5 moles of NaCl. The molar mass of NaCl is 58.44 g/mol.
Dimensional Analysis Steps:
- Multiply the given moles by 1 (to maintain the same value): 0.5 moles * 1
- Set up the conversion factor: (58.44 g/mol) / 1
- Multiply the original value by the conversion factor: (0.5 moles) * (58.44 g/mol) / 1
- Simplify the units: (0.5 moles) * (58.44 g) / (1 mole)
Result: 29.22 grams of NaCl
This calculation reveals that 0.5 moles of NaCl is equivalent to 29.22 grams of NaCl. By mastering the art of dimensional analysis, you unlock the ability to accurately convert between moles and grams, ensuring precision in your chemical calculations.