Fermentation: A Comprehensive Guide To Oxygen Deprivation And Glucose Metabolism
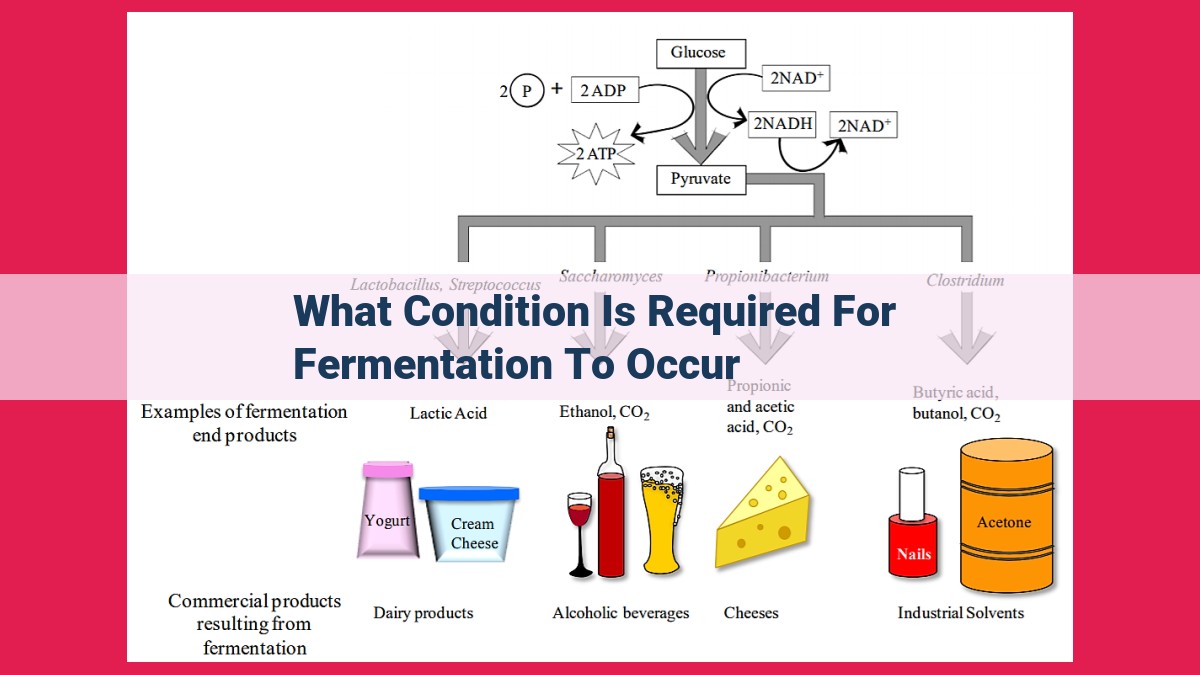
Essential for fermentation’s occurrence is oxygen deprivation, or anaerobic conditions, where no oxygen is present. In these conditions, glucose serves as the main energy source, and enzymes catalyze its breakdown. NAD+ acts as an electron acceptor, aiding energy-yielding reactions. Fermentation typically results in ethanol production as a byproduct. This process is prevalent in biological systems, especially microorganisms and muscle tissues, where oxygen is limited.
Fermentation: Unlocking Energy in the Absence of Oxygen
In the realm of life’s metabolic processes, fermentation stands as a crucial player, enabling living organisms to extract energy from fuel sources even when oxygen is scarce. Let’s delve deeper into the essential condition for fermentation: the deprivation of oxygen.
Anaerobic Environments: The Fermenter’s Haven
Fermentation is a distinctive metabolic pathway that occurs in the absence of oxygen (anaerobic conditions), unlike cellular respiration, which requires oxygen. This anaerobic environment creates a unique biochemical setting, setting the stage for the fermentation process to unfold.
Glucose: The Fuel of Fermentation
At the heart of fermentation lies glucose, a sugar molecule that serves as the primary substrate for this energy-yielding process. Glucose, when broken down in anaerobic conditions, provides the fuel that drives fermentation.
Enzymes: The Catalytic Masterminds
To facilitate the breakdown of glucose, a cast of enzymes steps into action. These protein catalysts orchestrate a series of chemical reactions, breaking down glucose into smaller molecules and ultimately yielding energy.
NAD+: The Electron Acceptor
In the fermentation process, NAD+ (nicotinamide adenine dinucleotide), a vital electron acceptor, plays a pivotal role. NAD+ acts as a shuttle, accepting electrons released during glucose breakdown, enabling the energy-yielding reactions of fermentation.
Ethanol: A Byproduct of Fermentation
A byproduct of fermentation, ethanol is a commonend product of this anaerobic process. Ethanol is produced when glucose is broken down in the absence of oxygen, serving as a reflection of the distinct metabolic pathway of fermentation.
Applications of Fermentation
Fermentation finds widespread applications across various biological systems. In microorganisms, fermentation enables survival and energy production in oxygen-limiting environments. In muscle tissues, fermentation plays a crucial role during strenuous activity, providing energy when oxygen supply is insufficient.
Fermentation, a metabolic process that occurs in the absence of oxygen, is a testament to life’s remarkable adaptability. It unveils the intricate interplay between anaerobic conditions, substrate availability, enzyme catalysis, and energy production. Fermentation’s significance extends to diverse biological systems, highlighting its fundamental role in sustaining life in varied and challenging environments.
**Fermentation: Unveiling the Secrets of Life in Oxygen-Deprived Environments**
In the realm of biology, fermentation stands out as a crucial process that allows life to thrive even in the absence of oxygen. This intriguing phenomenon occurs when microorganisms, such as yeast and bacteria, break down glucose to produce energy in oxygen-deprived environments.
What are Anaerobic Conditions?
Anaerobic conditions are characterized by the absence of oxygen. In such environments, organisms have adapted to utilize alternative mechanisms for energy production. Fermentation is one such mechanism that allows them to survive and function without the presence of oxygen.
Fermentation vs. Respiration
Respiration is a process that requires oxygen and produces a significant amount of energy in the form of ATP. In contrast, fermentation occurs in the absence of oxygen and yields a smaller amount of energy. While both processes utilize glucose as their primary substrate, the pathways they take are distinct.
The Role of Enzymes in Fermentation
Enzymes are protein catalysts that play a critical role in facilitating chemical reactions. In the case of fermentation, specific enzymes are responsible for breaking down glucose into pyruvate. This process generates ATP, the cell’s energy currency.
The Importance of NAD+
NAD+ (nicotinamide adenine dinucleotide) is an essential electron acceptor in fermentation. It undergoes a reduction-oxidation reaction, accepting electrons from glucose during its breakdown. This reaction helps regenerate NAD+, which is crucial for the continuation of fermentation.
Ethanol as a Byproduct
During fermentation, ethanol is produced as a byproduct of glucose breakdown. Ethanol is a volatile liquid that contributes to the distinctive flavors and aromas of fermented foods and beverages, such as bread, wine, and beer.
The Critical Role of Glucose in Fermentation
In the realm of biology, fermentation stands as a vital process that enables organisms to extract energy from glucose in the absence of oxygen. This anaerobic pathway holds immense significance in various biological systems, ranging from microorganisms to our own muscle tissues.
As the primary substrate for fermentation, glucose serves as the essential fuel that drives this energy-yielding reaction. When oxygen levels dwindle, cells switch to fermentation as a backup mechanism to obtain energy. During this process, glucose is broken down into various end products, including ethanol, lactate, and carbon dioxide. The specific end product varies depending on the organism and the type of fermentation.
The role of glucose in fermentation extends beyond its function as an energy source. It also plays a crucial role in the electron transfer reactions that occur during this process. As glucose is broken down, electrons are released and transferred to electron acceptors, such as NAD+. These reduced electron acceptors are then used in subsequent reactions to generate energy.
The importance of glucose in fermentation cannot be overstated. This sugar molecule is the cornerstone upon which this essential biological process rests. Without glucose, fermentation would not be possible, and cells would be unable to extract energy in the absence of oxygen. This underscores the critical role that glucose plays in sustaining life in a diverse range of organisms.
Role of Enzymes as Catalysts in Fermentation
In the realm of fermentation, enzymes play the role of unsung heroes, facilitating chemical reactions with remarkable efficiency. These protein catalysts exist in all living organisms, serving as the key players in countless biochemical processes. Their extraordinary ability to reduce the activation energy of reactions makes them indispensable for a wide range of biological functions, including fermentation.
Among the array of enzymes involved in fermentation, two stand out as crucial players:
- **_Hexokinase_:** The _gatekeeper_ of fermentation, this enzyme phosphorylates **D-glucose**, the starting substrate for the process. This initial phosphorylation effectively **traps** glucose within the cell, ensuring its availability for further metabolic reactions.
- **_Pyruvate decarboxylase_:** The _culprit_ behind ethanol production, this enzyme removes **carbon dioxide** from **pyruvate**, an intermediate product of glucose breakdown. The resulting **acetaldehyde** is subsequently reduced by **_alcohol dehydrogenase_** to yield **ethanol**, the **hallmark byproduct of fermentation**. This enzymatic conversion **regenerates NAD+**, an essential electron acceptor in fermentation.
These enzymes, working in concert, orchestrate the intricate biochemical dance that transforms glucose into ethanol, a byproduct of anaerobic metabolism. Their catalytic prowess enables organisms to derive energy from glucose in the absence of oxygen, a feat crucial for their survival in oxygen-deprived environments.
NAD+: The Crucial Electron Acceptor in Fermentation
In the absence of oxygen, cells employ fermentation, a unique process that enables them to extract energy from glucose. At the heart of this process lies a key player: NAD+ (nicotinamide adenine dinucleotide).
NAD+ serves as an indispensable electron acceptor during fermentation. As glucose undergoes breakdown, electrons are released. These electrons are captured by NAD+, which becomes reduced to NADH. This electron transfer is crucial for the energy-yielding reactions that ensue.
NADH, armed with electrons, participates in redox reactions, facilitating the transfer of electrons to other molecules. This electron flow drives the production of ATP, the cellular energy currency. Without NAD+, the energy extraction from glucose during fermentation would be severely hindered.
NAD+ acts as a cofactor, a helper molecule that enables enzymes to perform their catalytic functions. Specific enzymes in the fermentation pathway rely on NAD+ to facilitate the transfer of electrons and the generation of ATP.
By accepting electrons and facilitating redox reactions, NAD+ plays a pivotal role in the energy-yielding processes of fermentation. This electron acceptor ensures that cells can extract energy from glucose even in the absence of oxygen, a crucial adaptation for survival in diverse environments.
Ethanol’s Birth in the Realm of Anaerobic Fermentation
As we venture into the captivating world of fermentation, we uncover the secrets behind the transformation of glucose into diverse byproducts. Among these products, ethanol stands out as a star, playing a pivotal role in the anaerobic breakdown of glucose.
When the life-sustaining oxygen retreats from the scene, leaving biological systems starved for its presence, a remarkable metabolic shift takes place. Fermentation emerges as the hero, offering a lifeline to energy-dependent processes. In this oxygen-deprived environment, fermentation assumes the helm, enabling cells to continue extracting the precious energy they need.
Glucose, the universal energy currency, steps into the spotlight as the primary substrate for fermentation. It’s like the star ingredient in a recipe, providing the essential building blocks for the intricate cascade of chemical reactions that lie ahead. As glucose embarks on its anaerobic journey, it encounters a vital accomplice: enzymes. These molecular catalysts, the unsung heroes of biochemistry, orchestrate the intricate dance of chemical reactions, facilitating the breakdown of glucose into smaller molecules.
In the anaerobic realm, the electron acceptor of choice is not oxygen, but rather a molecule called NAD+. Like a trusty sidekick, NAD+ diligently accepts electrons from glucose, enabling the release of energy. As NAD+ becomes saturated with electrons, its transformation into NADH signals the initiation of a new dance, paving the way for the formation of ethanol.
Ethanol, the intoxicating byproduct of fermentation, emerges as a testament to the remarkable adaptability of life. Under anaerobic conditions, when the presence of oxygen dwindles, ethanol becomes the emblem of fermentation, a byproduct of glucose’s valiant struggle to sustain energy production.