Understanding The F Sublevel: Essential For Predicting Chemical Properties
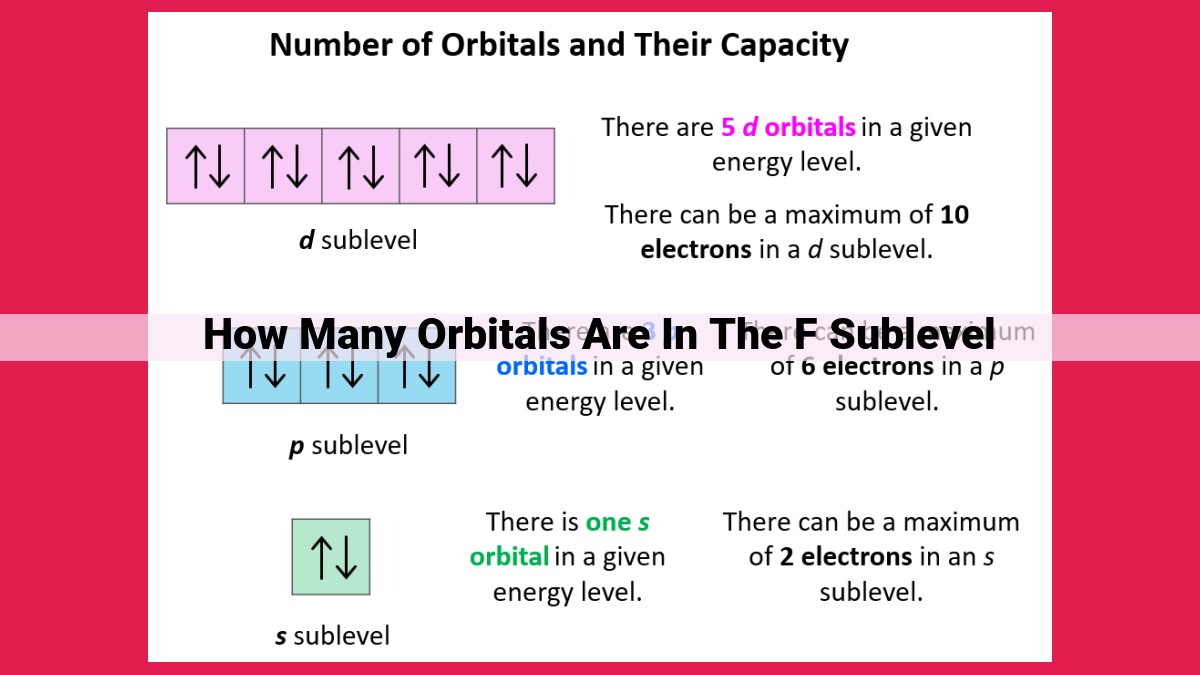
The f sublevel in an atomic orbital holds 7 orbitals, which is determined by the azimuthal quantum number (l) that defines the subshell. The f sublevel is part of the f block elements, characterized by the filling of electrons in the f orbitals. Understanding the number of orbitals in the f sublevel is crucial in chemistry as it helps predict the electronic configurations and chemical properties of elements, especially those in the f block.
Unveiling the Enigmatic F Sublevel: A Journey into the Realm of Atomic Orbitals
In the enigmatic world of chemistry, delving into the intricacies of atomic orbitals is paramount to unlocking the mysteries of chemical behavior. Among these orbitals, the f sublevel stands out with its unique properties and profound implications for our understanding of matter. Embark on a captivating odyssey as we unravel the secrets of the f sublevel, exploring its significance, structure, and role in shaping the chemical landscape.
Before venturing into the f sublevel, let us unravel the basics of atomic orbitals. These ethereal regions around an atom’s nucleus serve as probabilistic clouds, describing where electrons are most likely to reside. Each orbital is characterized by a set of quantum numbers, which serve as its unique address and determine its energy, shape, and orientation. The azimuthal quantum number (l), in particular, plays a pivotal role in determining the number of orbitals within a sublevel.
The f sublevel is the seventh and final sublevel in the hierarchy of atomic orbitals. Its l value of 3 dictates that it can accommodate a maximum of 7 orbitals, denoted as fx, fy, fz, fxy, fxz, fyz, and fxyz. These orbitals possess intricate shapes resembling cloverleaves and dumbbell-like structures, mirroring the unique quantum mechanical properties of the f sublevel.
Number of Orbitals in the f Sublevel
In the realm of chemistry and related fields, comprehending the number of orbitals within the f sublevel is paramount. Atomic orbitals, the designated regions where electrons reside, play a crucial role in determining the chemical behavior of elements.
Each sublevel, denoted by a letter (s, p, d, f), accommodates a specific number of orbitals. The azimuthal quantum number (l), one of the four quantum numbers that characterize electrons, dictates the number of orbitals within each sublevel.
For the f sublevel, designated by l = 3, the azimuthal quantum number indicates that it harbors seven orbitals. This distinctive sublevel arises from the unique shape and orientation of its orbitals, which are characterized by a complex three-dimensional geometry.
The f orbitals’ intricate shapes and energy levels influence the chemical bonding properties of f block elements. These elements, such as lanthanides and actinides, exhibit intriguing chemical behavior due to their partially filled f sublevels, leading to diverse applications in various fields.
Understanding the number of orbitals in the f sublevel provides a deeper insight into the electronic structure of atoms and the chemical properties of elements. This knowledge empowers scientists to unravel the complexities of chemical bonding, design new materials, and delve into cutting-edge research avenues.
The Enigmatic World of f Orbitals: Unraveling Their Shape and Significance in Chemical Bonding
In the captivating realm of chemistry and allied fields, understanding the number of orbitals in the f sublevel is an indispensable tool for deciphering atomic structures and chemical interactions. One might wonder, “Why does this matter?” Well, let’s paint a captivating tale to unveil the significance and intriguing nature of f orbitals.
Unveiling the Shape and Orientation of f Orbitals
Imagine a captivating dance of electrons within an atom, where each twirls in its designated orbital. f orbitals, belonging to the fifth energy level, possess a unique and intricate shape, unlike any other. They resemble intricate three-dimensional shapes, resembling lobes or petals extending in different directions.
The Symphony of f Orbitals in Chemical Bonding
Chemical bonding, the harmonious interplay between atoms, is intricately orchestrated by the overlap of atomic orbitals. f orbitals, with their extended lobes, can engage in directional bonding, forming strong bonds with other atoms. This exceptional characteristic makes them essential players in complex chemical reactions involving heavy elements like the actinides and lanthanides.
The Role of f Orbitals in the Identity of f Block Elements
f Orbitals lend their name to the enigmatic f block elements, which occupy a prominent place in the periodic table. These elements exhibit exceptional magnetic properties and form colorful compounds due to the presence of f electrons. Their unique electronic configurations, influenced by the f orbitals, determine their specific chemical behavior and reactivity.
The significance of understanding the number of orbitals in the f sublevel cannot be overstated. It unlocks the gateway to unraveling the intricate dance of electrons in atoms, enabling us to comprehend the formation of chemical bonds. Delving into the realm of f orbitals, their peculiar shapes, and their vital role in chemistry unveils a captivating tale of scientific wonder, empowering us to navigate the vast expanse of chemical reactions.
Unveiling the Enigma of F Block Elements
In the realm of chemistry, the arrangement of electrons within atoms plays a pivotal role in shaping their properties. Among the various electron sublevels, the f sublevel holds a special significance, particularly for a group of elements known as the f block elements. These intriguing elements possess unique chemical characteristics that stem from the peculiarities of their electronic configurations.
Defining the F Block Elements
The f block elements encompass the elements from lanthanum to lutetium, along with actinium and thorium. Unlike other elements, their defining feature lies in the presence of electrons occupying f orbitals, which are the outermost orbitals in their electronic configurations.
Electronic Configuration and its Significance
The electronic configuration of f block elements is marked by the systematic filling of f orbitals, progressively from 4f for lanthanum to 5f for lutetium and actinium, and 6f for thorium. This gradual filling of f orbitals gives rise to their exceptional chemical properties.
Unique Chemical Properties
F block elements exhibit a remarkable range of chemical properties that set them apart from other elements. These properties include:
- Variable Oxidation States: F block elements exhibit multiple oxidation states, owing to the availability of multiple f electrons that can be involved in chemical reactions.
- Formation of Stable Complexes: F block elements form stable complexes with various ligands, displaying a rich coordination chemistry due to the availability of vacant f orbitals for bonding.
- Magnetic Properties: Many f block elements exhibit paramagnetic behavior as a result of the presence of unpaired electrons in their f orbitals.
- Lanthanide Contraction: The gradual decrease in atomic size from lanthanum to lutetium, known as lanthanide contraction, significantly influences the chemical properties of these elements.
- Actinide Series: The actinide elements exhibit radioactive properties and are involved in nuclear reactions, making them significant in the fields of nuclear chemistry and energy production.
F block elements stand as a testament to the intricate interplay between electronic configurations and chemical properties. Their unique characteristics, arising from the occupation of f orbitals, make them indispensable in various scientific disciplines, including chemistry, materials science, and nuclear physics. Understanding the number of orbitals in the f sublevel provides a fundamental framework for comprehending the behavior and applications of these fascinating elements.
Related Concept: Atomic Orbitals
- Provide an overview of different types of atomic orbitals (s, p, d, f).
- Discuss the quantum numbers associated with atomic orbitals.
Understanding the Number of Orbitals in the f Sublevel
In the realm of chemistry and allied sciences, comprehending the number of orbitals in the f sublevel holds immense significance. Orbitals, defined by quantum mechanics, depict the probabilistic regions around an atomic nucleus where electrons are likely to be found. Each sublevel, including the f sublevel, contains a specific number of orbitals that play a crucial role in determining the chemical properties of elements.
Number of Orbitals in the f Sublevel
The f sublevel is a unique set of seven orbitals characterized by their distinctive shape and orientation. Designated as f₁ through f₇, these orbitals exist in the sixth and seventh energy levels of atoms. The number of orbitals in a sublevel is determined by the azimuthal quantum number (l), which describes the shape of the orbital. For the f sublevel, l = 3, which indicates a more complex shape compared to other sublevels.
Shapes and Roles of f Orbitals
f orbitals possess a complex, three-dimensional shape with multiple lobes. They exist in two distinct sets: three orbitals aligned along the Cartesian axes and four orbitals positioned between the axes. These orbitals play a vital role in chemical bonding, particularly in coordination complexes where they can form strong covalent bonds with ligands.
f Block Elements and Their Significance
Elements with electrons in the f sublevel are known as f block elements. These elements belong to Group 3 in the periodic table and include lanthanides and actinides. f block elements exhibit unique chemical properties due to the presence of f electrons. Their electronic configurations, characterized by partially filled f orbitals, influence their magnetic and spectroscopic properties.
An Overview of Different Types of Atomic Orbitals
Atomic orbitals come in various types, each with its own shape and energy level. The s orbitals are spherical, the p orbitals are dumbbell-shaped, and the d orbitals have more complex shapes. The f orbitals, with their even more intricate geometry, complete the series of atomic orbitals.
Quantum Numbers and Atomic Orbitals
Four quantum numbers (n, l, ml, ms) collectively describe the energy and shape of atomic orbitals. The principal quantum number (n) represents the energy level, the azimuthal quantum number (l) signifies the shape, the magnetic quantum number (ml) specifies the orientation in space, and the spin quantum number (ms) indicates the electron’s spin. These quantum numbers play a fundamental role in understanding the behavior of electrons within atoms.
Understanding the number of orbitals in the f sublevel is essential for comprehending the chemical properties of elements and the behavior of electrons in atoms. This knowledge underlies various branches of chemistry and physics, including spectroscopy, nuclear chemistry, and materials science. By incorporating this concept into our understanding, we gain a deeper appreciation for the intricate nature of the atomic world.
Related Concept: Quantum Numbers
- Explain the four quantum numbers (n, l, ml, ms) and their significance.
- Describe how quantum numbers determine the energy and shape of orbitals.
Quantum Numbers: Unraveling the Mystery of Atomic Orbitals
In the captivating realm of chemistry and its allied disciplines, understanding the number of orbitals in a given sublevel is a cornerstone of knowledge. One such sublevel of particular interest is the f sublevel, which plays a pivotal role in the study of chemistry. But before delving into its intricacies, let us first unravel the secrets of atomic orbitals and the enigmatic quantum numbers that govern their existence.
Every atom possesses a nucleus surrounded by a cloud of electrons that occupy specific regions known as orbitals. These orbitals are characterized by four fundamental quantum numbers:
- Principal quantum number (n): This number represents the energy level or shell in which the electron resides.
- Azimuthal quantum number (l): This number describes the shape of the orbital, with values ranging from 0 to n-1. For the f sublevel, l = 3.
- Magnetic quantum number (ml): This number determines the orientation of the orbital in space, with values ranging from -l to +l. For the f sublevel, ml can have seven possible values, leading to the presence of seven orbitals.
- Spin quantum number (ms): This number represents the intrinsic spin of the electron, which can be either “up” or “down.”
These quantum numbers work in concert to determine the energy, shape, and orientation of atomic orbitals. They form the essential framework upon which the electronic structure of atoms and molecules is built. By harnessing the power of quantum numbers, chemists can unravel the complex tapestry of atomic and molecular interactions, leading to a deeper understanding of the fundamental building blocks of our universe.