Exothermic Reactions: Temperature Increase And Equilibrium Shifts Explained
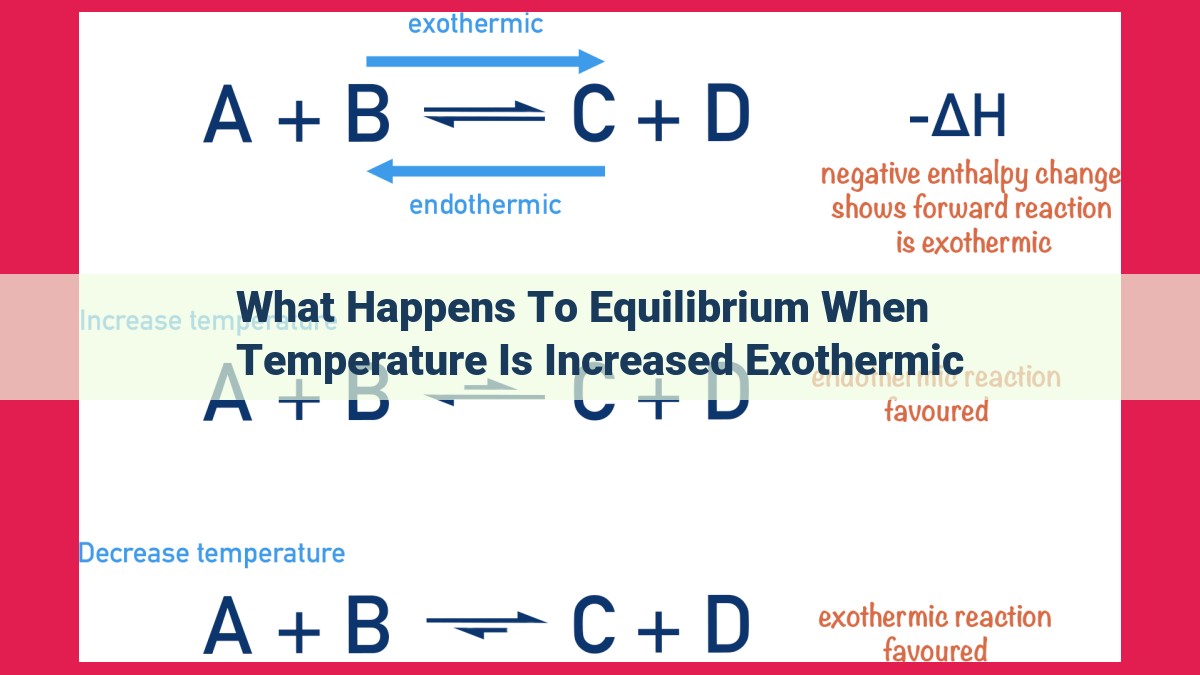
When temperature increases in an exothermic reaction (heat is released), equilibrium shifts to relieve the stress created by the added heat. Le Chatelier’s principle predicts that the system will shift in a direction that consumes the added energy. In an exothermic reaction, this means a shift towards the product side, since the forward reaction (products forming) is exothermic and absorbs the added heat, restoring equilibrium. This shift favors the formation of products, emphasizing the relationship between exothermic reactions and product favorability.
Le Chatelier’s Principle: A Guide to Understanding Equilibrium Shifts in Exothermic Reactions
In the realm of chemistry, the concept of chemical equilibrium plays a pivotal role in shaping the behavior of countless reactions. Chemical equilibrium occurs when the forward and reverse reactions of a particular process happen at the same rate, resulting in a seemingly unchanging state. Understanding how to manipulate this equilibrium is key to harnessing the power of chemical reactions.
One of the most powerful tools we have for understanding equilibrium shifts is Le Chatelier’s principle. This principle states that if a stress is applied to a system at equilibrium, the system will shift in a way that relieves that stress. Stress can take many forms, including changes in temperature, concentration, or volume.
Le Chatelier’s Principle in Action: Exothermic Reactions
Exothermic reactions are reactions that release heat energy into their surroundings. When heat is added to an exothermic reaction, Le Chatelier’s principle predicts that the reaction will shift in a direction that absorbs heat. This makes sense intuitively: adding heat to an exothermic reaction is like putting more logs on a fire—the reaction speeds up and more heat is released.
To illustrate this, let’s consider the following exothermic reaction:
A + B <=> C + heat
If we add heat to this reaction, Le Chatelier’s principle tells us that the reaction will shift to the left, absorbing the added heat. This is because the reverse reaction (C → A + B) consumes heat, so shifting in that direction relieves the stress of the added heat.
Shifting the Equilibrium Towards the Product Side
The implication of this shift is that adding heat to an exothermic reaction favors the production of reactants (A and B) over products (C). This is because the equilibrium position moves to the left, where there are more reactants and less products.
This relationship between exothermic reactions and product favorability is crucial for understanding and controlling chemical systems. For example, if you want to maximize the yield of a product in an exothermic reaction, you can increase the temperature to shift the equilibrium towards the product side.
Le Chatelier’s principle is a powerful tool for understanding and predicting equilibrium shifts in chemical reactions. By applying this principle, we can manipulate the outcome of reactions and optimize them for specific purposes. The case of exothermic reactions demonstrates how Le Chatelier’s principle can be used to favor the production of desired products by manipulating temperature, offering valuable insights for chemists and researchers alike.
Le Chatelier’s Principle: A Guide to Predicting Equilibrium Shifts
Chemical equilibrium is a state of dynamic balance where the concentrations of reactants and products in a reaction remain constant over time. Understanding how to predict shifts in equilibrium is crucial for comprehending chemical system behavior. Here’s where Le Chatelier’s principle comes into play.
Le Chatelier’s principle states that if a stress is applied to a system at equilibrium, the system will shift in a direction that relieves that stress. This principle provides a powerful tool for predicting equilibrium shifts.
Imagine a closed container filled with a reacting gas mixture at equilibrium. If we suddenly increase the temperature of the container, the system will experience a stress. According to Le Chatelier’s principle, the system will shift in a direction that relieves this stress.
For an exothermic reaction, the release of heat is a stress on the system. To relieve this stress, the system will shift towards the reactant side, consuming heat and restoring equilibrium at a lower temperature. This is because the reverse reaction (exothermic) absorbs heat, effectively reducing the added heat stress.
Conversely, if we decrease the temperature of the container, the system will shift towards the product side. By releasing heat, the exothermic reaction counteracts the lowered temperature, restoring equilibrium at a higher temperature. This shift favors the formation of products, as the forward reaction (exothermic) releases heat and offsets the cooling effect.
Le Chatelier’s principle is a valuable tool for chemists to predict the behavior of chemical systems under different conditions. By understanding how equilibrium shifts relieve stress, we can make informed predictions about the direction and extent of reactions.
Exothermic Reactions: Unveiling the Effects of Temperature
In the realm of chemistry, reactions are often classified as either exothermic or endothermic. Exothermic reactions are those that release heat energy into their surroundings. This energy release is caused by the breaking of chemical bonds in the reactants and the formation of new, stronger bonds in the products.
Exothermic Reactions and Heat Addition
When heat is added to an exothermic reaction, it intensifies the process. The added energy facilitates the breaking of more reactant bonds, leading to an increased production of products. This effect is in stark contrast to endothermic reactions, which require heat to proceed.
Delving into Le Chatelier’s Principle
According to Le Chatelier’s principle, when stress is applied to a system at equilibrium, the system shifts in a direction that counteracts or relieves the stress. In the case of exothermic reactions, the addition of heat acts as a stress.
How Heat Shifts Equilibrium
In exothermic reactions, the system relieves the stress of added heat by shifting towards the reactant side. This shift is driven by the exothermic nature of the reaction, as the breaking of reactant bonds favors the release of heat. By producing more reactants, the system reduces the concentration of products, thereby decreasing the heat released.
Favoring the Product Side
While the initial equilibrium shift is towards the reactant side, it is important to note that exothermic reactions ultimately favor the product side. The release of heat drives the formation of stronger product bonds, ultimately leading to a higher concentration of products at the new equilibrium position.
Understanding the behavior of exothermic reactions under varying temperatures is crucial for predicting chemical system responses. Le Chatelier’s principle provides a valuable framework for predicting equilibrium shifts, particularly in the context of energy exchange. By unraveling the effects of heat addition, we can optimize chemical processes and harness the power of exothermic reactions in practical applications.
Equilibrium Shift in Exothermic Reactions: Shifting the Balance with Heat
Imagine you have a chemical reaction that gives off heat, known as an exothermic reaction. Think of it as a cozy campfire that releases warmth into the air. Just like that campfire, when you add more heat to an exothermic reaction, it’s like adding more fuel to the flames. And just as the fire’s intensity increases, so too does the reaction’s tendency to shift its equilibrium in a particular direction.
Le Chatelier’s Principle: The Equilibrium Guardian
Le Chatelier’s principle is like a wise old chemist who predicts how a reaction will respond to external disturbances, like the addition of heat. It says that when you apply stress (like adding heat) to a reaction at equilibrium, the reaction will shift in a direction that relieves that stress.
Stress Relief for Exothermic Reactions
So, how does this principle play out in exothermic reactions? When you add heat to an exothermic reaction, it’s like piling more wood onto a campfire. The reaction responds by trying to get rid of the excess heat, like a campfire releasing more flames and smoke. And guess what? It does this by shifting the equilibrium towards the reactant side. That’s right, the opposite direction of what you might expect.
Relieving Heat Stress
This shift relieves the heat stress because it decreases the number of moles of products, which are responsible for releasing heat. By shifting towards the reactant side, the reaction essentially absorbs the added heat and cools down, just like adding more wood to a campfire can help control its temperature.
Product Favorability: The Heat’s Ally
While the equilibrium shift towards the reactant side may seem counterintuitive, it actually favors the production of more reactants. This is because in exothermic reactions, the products are less stable than the reactants and are more likely to break down and release heat. By shifting the equilibrium towards the reactants, the reaction helps to maintain a more stable system. In other words, the addition of heat promotes the formation of the more stable reactants over the less stable products.
Understanding equilibrium shifts in exothermic reactions is essential for predicting chemical system behavior. Le Chatelier’s principle provides a powerful tool to anticipate how a reaction will respond to changes in conditions, allowing chemists to optimize reactions and control product formation. So next time you’re caught in the heat of an exothermic reaction, remember that Le Chatelier’s principle is your ally, guiding you towards a more balanced equilibrium.
How Increased Temperature Favors the Product Side in Exothermic Reactions
Le Chatelier’s principle predicts how chemical systems respond to changes in their conditions, such as temperature or concentration. When a system is in equilibrium, these changes can shift the balance of reactants and products.
In an exothermic reaction, heat is released as products are formed. Le Chatelier’s principle tells us that adding heat to an exothermic reaction will shift the equilibrium towards the reactant side. This is because the system tries to relieve the stress caused by the added heat by forming more reactants, which consume heat.
However, if the temperature is increased in an exothermic reaction, a different effect is observed. In this case, the equilibrium shifts towards the product side. This is because the increased temperature favors the product side.
The reason for this shift can be understood in terms of entropy. Entropy is a measure of disorder in a system. In general, reactions that form more moles of gas or more disordered products have a higher entropy. In an exothermic reaction, the products are usually more ordered than the reactants. This means that the reaction has negative entropy change.
When temperature is increased, entropy becomes more important. The system tries to increase its entropy by favoring the products that have higher entropy. This is why the equilibrium shifts towards the product side in an exothermic reaction when temperature is increased.
The relationship between exothermic reactions and product favorability is important to understand because it can be used to predict the behavior of chemical systems. For instance, if you want to increase the yield of a product in an exothermic reaction, you can do so by increasing the temperature. This strategy is commonly used in industrial chemistry.