Exothermic Phase Transitions: Unraveling Heat Release In Melting, Boiling, And Sublimation
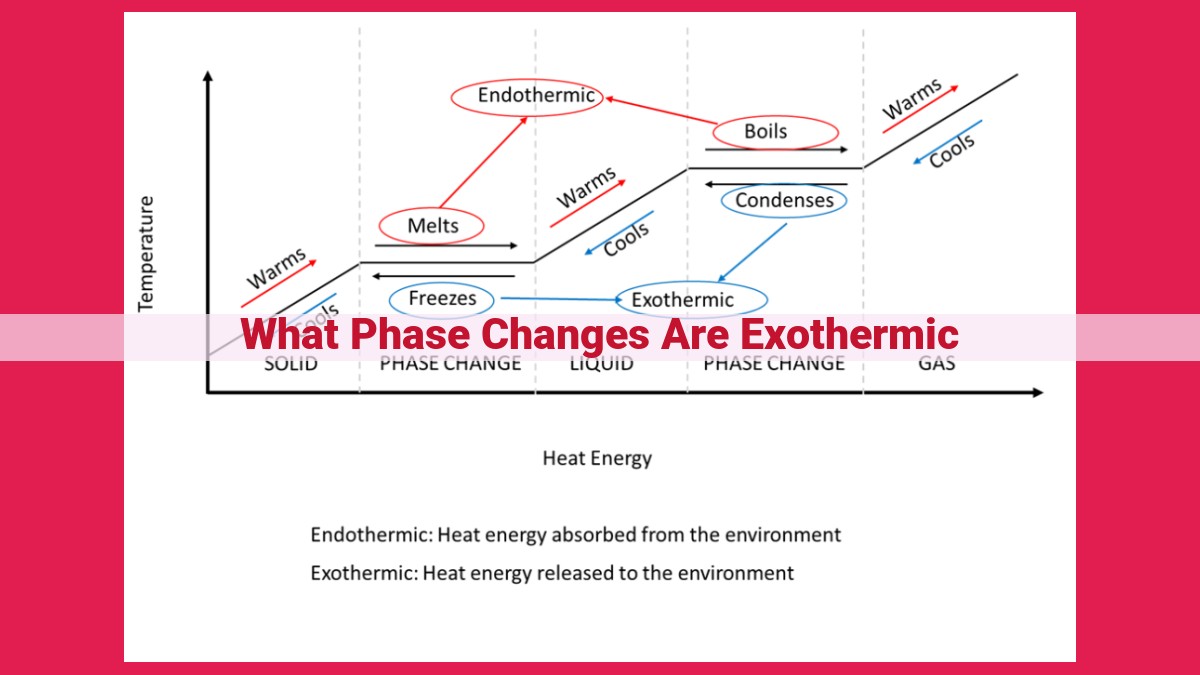
Phase changes involving the transition from a solid to a liquid or a gas (melting, boiling, sublimation) are exothermic, meaning they release heat. During melting, heat breaks down the ordered structure of a solid, causing its particles to become more disordered in the liquid state. Similarly, in boiling and sublimation, heat increases molecular kinetic energy, weakening intermolecular bonds and allowing particles to transition into the gas phase. The release of heat in these processes is due to the decrease in potential energy as particles move apart and gain more freedom of movement.
Phase Changes: Exothermic Processes Unveiled
Phase changes, those mesmerizing transformations that materials undergo, are a captivating dance of energy transfer. When a material glides from one state to another—solid to liquid, liquid to gas, and beyond—heat plays a pivotal role. In this realm of molecular ballet, some phase changes release heat, while others absorb it. Let’s focus on the fascinating exothermic processes, where heat is unleashed upon the surroundings.
Exothermic Phase Changes: Unveiling the Energy Release
Exothermic phase changes are like small exothermic fireworks, releasing heat into the environment as a material transforms. This heat, often harnessed for practical applications, stems from the breakdown of intermolecular forces, the forces that hold molecules together. As a material transitions from a more ordered state to a less ordered one, these forces weaken or break, releasing their stored energy as heat.
Melting: Heat Liberating Solid to Liquid
Picture this: a lump of ice, a solid masterpiece. As heat gently embraces the ice, it starts to melt, transitioning from a rigid solid to a flowing liquid. This transformation is an exothermic process. Heat breaks apart the strong bonds that hold the water molecules in their geometric lattice, freeing them to move more freely. The release of heat during melting has practical implications, as seen in ice packs that absorb heat to cool injuries.
Boiling: Heat Absorption for Liquid to Gas
Now, let’s turn up the heat and observe boiling, where a liquid like water transforms into a gaseous state. Heat plays a critical role here, causing water molecules to vibrate more vigorously, breaking the intermolecular bonds that hold them together. These freed molecules escape into the air as vapor, absorbing heat from the surroundings. Boiling is an endothermic process, but since we are focusing on exothermic changes, let’s not dwell on it for now.
Summary and Significance
Phase changes are ubiquitous in our world, from the melting of glaciers to the formation of clouds to the operation of air conditioners. Exothermic phase changes, in particular, are fascinating processes that release heat, often harnessed for practical applications. Understanding these concepts helps us appreciate the intricate interplay of energy and matter in the world around us.
Melting: An Exothermic Transformation
As we delve into the realm of phase changes, we encounter the fascinating phenomenon of melting, a transformation that unveils the captivating power of heat in reshaping matter. Melting is the journey from a solid’s rigid embrace into the fluidity of a liquid.
Unlike everyday experiences where heat typically flows from a hotter to a colder object, the process of melting defies this convention. Here, heat is absorbed by the solid, triggering an internal transformation. As heat penetrates a solid, it energizes the molecules, causing them to vibrate with greater intensity.
This increased molecular motion disrupts the rigid bonds that hold the molecules in place, gradually undermining the solid’s crystalline structure. As the structure weakens, molecules break free from their fixed positions and gain the freedom to move about more freely, marking the birth of a liquid.
During this transition, the absorbed heat is not simply stored but is instead consumed in overcoming the forces that confine the molecules. This process, known as latent heat of fusion, embodies the energy required to break the bonds between molecules and allow them to flow.
The exothermic nature of melting reveals itself as the liquid cools. As the molecules lose energy, they slow down and, guided by the forces of intermolecular attraction, reform bonds,重新形成键, reverting back to a solid state. This exothermic process releases the heat that was initially absorbed during melting, providing a glimpse into the energy dynamics that govern phase changes.
Boiling: A Heat-Absorbing Phase Change
In our exploration of phase changes, we delve into the captivating realm of boiling, a phenomenon marked by a dramatic exothermic transformation. As we immerse ourselves in this process, imagine a bubbling cauldron of water, its contents dancing and transforming right before our very eyes.
Boiling occurs when a liquid reaches its boiling point, which is the temperature at which its vapor pressure equals the pressure of the surrounding environment. At this critical point, the liquid’s molecules gain sufficient kinetic energy, enabling them to break free from the intermolecular bonds that hold them together.
As heat is relentlessly applied, the molecules’ kinetic energy soars, colliding with each other with increasing vigor. These collisions weaken the intermolecular bonds, creating gaps and pockets of vapor within the liquid. When these vapor pockets accumulate enough energy, they burst forth as bubbles, propelling themselves to the liquid’s surface.
Upon reaching the surface, the vapor bubbles explode, releasing molecules that transition into the gas phase. This exothermic process absorbs heat from the surroundings, causing a dip in temperature. The liquid’s temperature remains constant at the boiling point until all of the liquid has transformed into vapor.
In essence, boiling is a cooling process that absorbs heat from its surroundings. This principle finds practical application in various cooling systems, such as evaporative coolers and air conditioners. By harnessing the exothermic nature of boiling, these devices effectively remove heat from the environment, creating a more comfortable and refreshing atmosphere.
Condensation: The Exothermic Transformation from Gas to Liquid
Condensation is the phase change that occurs when a gas transforms into a liquid, releasing heat as it does. This process is the opposite of evaporation and plays a significant role in the Earth’s water cycle and other natural phenomena.
Defining Condensation and Its Energy Release
Condensation occurs when a gas cools to a temperature below its boiling point. As the gas molecules lose kinetic energy, they slow down and become closer together. This weakening of intermolecular bonds allows the molecules to form liquid bonds, resulting in the condensation of the gas into a liquid.
Cooling and Molecular Interactions
During condensation, the temperature of the gas decreases, reducing the kinetic energy of its molecules. This loss of energy weakens the intermolecular forces that keep the gas molecules separated. As the molecules lose mobility, they become more attracted to each other, and liquid bonds start to form.
Example: Formation of Clouds
One of the most visible examples of condensation is the formation of clouds. As warm, moist air rises in the atmosphere, it cools, causing the water vapor in the air to condense into tiny water droplets. These droplets form visible clouds that can lead to precipitation.
Freezing: Exothermic Formation of Solids
- Define freezing and explain its exothermic nature.
- Describe how cooling causes liquid molecules to lose kinetic energy and form a solid structure.
Freezing: The Exothermic Transformation of Liquids to Solids
As we witness the transformations of matter, we often encounter phase changes, where a substance transitions from one state to another. One such transformation is freezing, where a liquid cools and solidifies. This process, surprisingly, is accompanied by a release of heat, making freezing an exothermic process.
Understanding the Nature of Freezing
At the molecular level, freezing involves the loss of kinetic energy by liquid molecules, causing them to slow down and lose their ability to retain their liquid state. As the temperature drops, these molecules lose even more energy, colliding less frequently and forming stronger bonds with each other. This arrangement of molecules results in a solid structure, characterized by a fixed shape and volume.
The Exothermic Nature of Freezing
The process of freezing releases heat because the newly formed solid structure is more ordered than the liquid state. This increase in orderliness results in a decrease in the system’s entropy, which is the measure of disorder. According to the second law of thermodynamics, when entropy decreases, energy must be released, hence the exothermic nature of freezing.
Applications of Freezing
The exothermic nature of freezing finds numerous practical applications. For instance, refrigerators utilize this process to remove heat from food, preserving it. Ice packs also rely on freezing to provide cooling relief, as the melting ice absorbs heat from the body. Additionally, freezing plays a critical role in food preservation, slowing down chemical reactions and preventing spoilage.
Sublimation: The Ethereal Transformation of Solids into Gases
Sublimation, an exothermic phase change, is a mesmerizing transformation where solids bypass the liquid state and directly ascend into the realm of gases. This intriguing process is driven by the dance of heat and intermolecular forces.
Unveiling the Exothermic Nature of Sublimation
When heat embraces a solid, it infuses its molecules with an elevated kinetic energy, stirring them into a fervor of motion. This surge of energy weakens the intermolecular forces that bind the molecules together, causing their grip to loosen. As the bonds become more feeble, the molecules break free from the rigid lattice structure that once held them captive.
The Journey of Molecules: From Solid to Gas
With newfound freedom, the liberated molecules embark on a journey to the gaseous realm. Sublimation unfolds as these molecules overcome the remaining intermolecular forces and escape into the open expanse of space. The exothermic nature of this process stems from the energy released as the molecules break away from their solid bonds and assume their gaseous state.
Applications of Sublimation: From Nature to Industry
Sublimation is a ubiquitous phenomenon with far-reaching applications in both nature and industry. In nature, it plays a pivotal role in the formation of snow crystals and the enigmatic dry ice that billows from frozen surfaces. Industrially, sublimation is employed in the purification of substances and the production of specialized materials, such as freeze-dried foods and pharmaceuticals.
Sublimation stands as a testament to the delicate balance between heat and intermolecular forces. This enchanting phase change offers a glimpse into the molecular realm, where exothermic processes orchestrate the ethereal transformation of solids into gases. From the snowflake’s intricate dance to the industrial applications that shape our world, sublimation remains a captivating phenomenon that weaves its magic throughout the universe.
Deposition: Gas Condensation to Solids
- Define deposition and explain its exothermic nature.
- Describe how cooling causes gas molecules to lose kinetic energy and form a solid structure directly.
Deposition: When Gas Molecules Undergo an Exothermic Transformation to Form Solids
In the enchanting world of matter, phase changes are akin to magical transformations, where substances effortlessly switch between solid, liquid, and gas states. Among these transitions, deposition stands out as a captivating process where gas molecules perform an extraordinary feat: they bypass the liquid phase altogether and morph directly into solid form.
At the heart of this remarkable process lies an intricate dance between temperature and molecular energy. As gas molecules encounter a cooling environment, their kinetic energy inevitably diminishes, causing their once-energetic movements to slow down. This loss of energy weakens the invisible bonds that held them apart, allowing them to draw closer and form solid structures.
Imagine snowflakes, those delicate crystals that adorn the winter landscape. They are quintessential examples of deposition. As water vapor in the air encounters the frigid temperatures of the atmosphere, its molecules shed their gaseous guise and coalesce into intricate, solid flakes.
The exothermic nature of deposition stems from the release of energy as gas molecules condense into solids. This energy, once stored within the molecules’ motion, is now freed as they settle into their new, more organized arrangement.
Deposition is a ubiquitous phenomenon that plays a vital role in nature and technology alike. From the formation of frost on a cold windowpane to the creation of specialized materials in industry, this process showcases the versatility and transformative power of matter.