Understanding Exergonic And Endergonic Reactions: Energy, Spontaneity, And Entropy
Exergonic reactions release energy and are spontaneous, while endergonic reactions absorb energy and are nonspontaneous. Exergonic reactions have a negative change in Gibbs free energy (ΔG), while endergonic reactions have a positive ΔG. Spontaneity is also linked to entropy, which measures disorder; reactions that increase disorder tend to be spontaneous. These concepts predict the directionality and spontaneity of chemical reactions and have practical applications in chemistry and biology.
Exergonic vs. Endergonic Reactions: Understanding Chemical Energy Flow
In the world of chemistry, reactions play a crucial role in shaping our surroundings. These reactions involve the exchange of energy, leading to the formation of new substances. Exergonic and endergonic reactions represent two distinct types of energy exchange that dictate the spontaneity and directionality of chemical reactions.
Exergonic Reactions: Releasing Energy
Exergonic reactions are like a downhill slide in the energy landscape. They release energy as they proceed, producing products with lower energy than the reactants. Think of a rock rolling down a hill; the rock releases its gravitational potential energy as it falls. Similarly, in exergonic reactions, the release of energy often manifests as heat, light, or electrical energy.
Spontaneity of Exergonic Reactions:
Exergonic reactions are spontaneous, meaning they occur naturally without any external input. The energy released by the reaction drives the process forward, making it self-sustaining. Imagine a stream flowing downhill; the force of gravity pulls the water, causing it to flow without the need for external energy.
Endergonic Reactions: Absorbing Energy
In contrast to exergonic reactions, endergonic reactions are like climbing uphill. They require an input of energy to proceed, forming products with higher energy than the reactants. It’s like a person pushing a heavy object up a slope; they must exert energy to overcome the opposing gravitational force.
Nonspontaneity of Endergonic Reactions:
Endergonic reactions are nonspontaneous, meaning they do not occur naturally without a continuous energy supply. The energy required must be provided from an external source, such as heat, light, or electrical energy. Think of a pump pushing water uphill; the pump provides the energy to overcome the opposing force of gravity.
**Entropy and Spontaneity**
Entropy, the Measure of Disorder
Imagine a perfectly organized room, with everything in its place. Now, picture the same room after a toddler’s playtime. Chaos reigns supreme, and disorder has taken over. This mess is a perfect example of entropy, a measure of the randomness or disorder of a system. The higher the entropy, the more chaotic the system.
Entropy’s Influence on Reactions
In chemical reactions, entropy plays a crucial role in determining spontaneity. Spontaneous reactions are those that occur naturally, without any external input of energy. Nonspontaneous reactions, on the other hand, require energy to proceed.
Entropy favors réactions that lead to more **disordered states. This means that reactions that produce products with higher entropy will be more spontaneous. For example, the reaction of sugar dissolving in water is spontaneous because the sugar molecules spread out and become more disordered in solution.
The Role of Entropy in Predicting Spontaneity
In thermodynamics, the concept of Gibbs free energy is used to predict the spontaneity of réactions. The change in Gibbs free energy (ΔG) is a measure of the energy available to do work. Negative ΔG values indicate spontaneous reactions, while positive ΔG values indicate nonspontaneous reactions.
Entropy’s contribution to ΔG
Entropy, along with enthalpy, plays a significant role in determining ΔG. A negative entropy change (ΔS < 0) indicates that the reaction is becoming more ordered, which makes it less spontaneous. Conversely, a positive entropy change (ΔS > 0) indicates that the reaction is becoming more disordered, which makes it more spontaneous.
Understanding entropy and its impact on spontaneity is essential for predicting the directionality and spontaneity of chemical reactions. By considering the change in entropy along with other factors, scientists can gain valuable insights into the behavior of systems, both in the laboratory and in the natural world.
Gibbs Free Energy: Unveiling the Secrets of Spontaneous Reactions
In the realm of chemistry, spontaneous reactions hold a special fascination. They’re like the star performers in a chemical dance, unfolding effortlessly, without any external assistance. But what’s the secret behind their grace and spontaneity? That’s where Gibbs free energy steps onto the stage.
Gibbs free energy (G) is a measure of the energy available to do useful work in a chemical system. It’s a delicate balance between two opposing forces: enthalpy (H), which represents the system’s internal energy, and entropy (S), a measure of the system’s disorder and chaos.
When a reaction is spontaneous, it releases Gibbs free energy (ΔG < 0). This negative ΔG indicates that the products of the reaction have lower energy than the reactants, and the reaction can proceed without any external input of energy. It’s like water flowing downhill, seeking the lowest energy state.
Conversely, non-spontaneous reactions require an input of energy (ΔG > 0). In these reactions, the products have higher energy than the reactants, and the reaction won’t occur spontaneously. It’s like trying to climb a hill without any help.
ΔG, the driving force of spontaneity
The value of ΔG not only reveals the spontaneity of a reaction but also its directionality. A positive ΔG indicates that the reaction is non-spontaneous and will only proceed if energy is supplied from an external source. On the other hand, a negative ΔG indicates that the reaction is spontaneous and will proceed without any external input of energy.
The importance of Gibbs free energy
Understanding Gibbs free energy is crucial for predicting the spontaneity and directionality of chemical reactions. This knowledge finds applications in diverse fields, including:
- Chemical engineering: Designing processes that maximize energy efficiency and yield
- Biochemistry: Understanding metabolic pathways and energy transfer in living cells
- Environmental chemistry: Predicting the fate and transport of pollutants
In conclusion, Gibbs free energy is a powerful tool that allows us to unravel the secrets of spontaneous reactions. By measuring ΔG, we can determine whether a reaction will proceed spontaneously or not, as well as its directionality. Understanding Gibbs free energy is essential for a wide range of applications, making it a cornerstone of chemical and biological sciences.
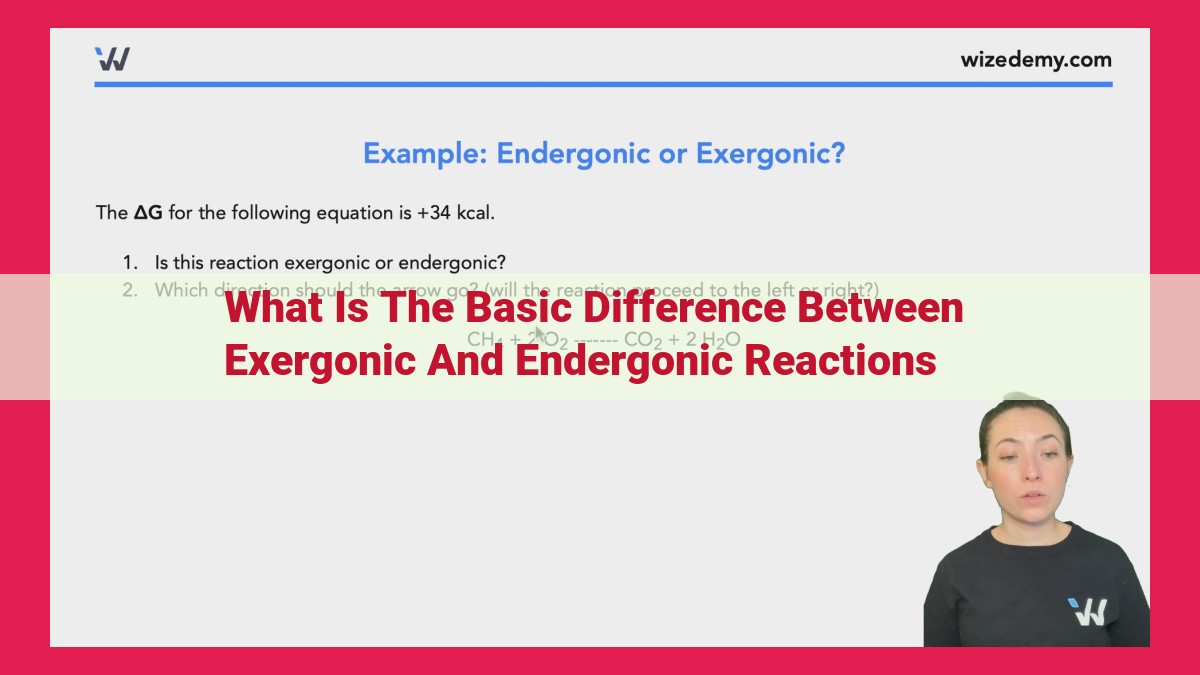
ΔG and Reaction Directionality
The free energy change, represented by ΔG, is a crucial parameter that determines the direction and spontaneity of chemical reactions. It measures the difference between the initial and final states of a system, providing insights into the energy availability for a given reaction.
Positive ΔG values indicate that the reaction is nonspontaneous. This means the reaction proceeds from a lower energy state to a higher energy state, requiring an input of energy to occur. In other words, the reaction is endergonic.
On the other hand, negative ΔG values signify spontaneous reactions. These reactions flow from a higher energy state to a lower energy state, releasing energy in the process. Thus, they are considered exergonic.
By assessing the sign of ΔG, scientists can predict the direction and spontaneity of chemical reactions. This knowledge is essential in understanding various processes in chemistry, biology, and other disciplines that involve energy transformations.
Distinguishing Exergonic and Endergonic Reactions: Unraveling the Key Differences
In the realm of chemistry, understanding the nature of reactions is crucial. Two fundamental types of reactions, exergonic and endergonic, differ significantly in their energy exchange, spontaneity, and impact on entropy. Let’s dive into the key distinctions between these reactions to gain a deeper understanding of their behavior.
Energy Exchange: A Tale of Release and Absorption
Exergonic reactions, as their name suggests, release energy during the reaction process. Think of them as energetic exhalations, releasing energy into the surroundings. In contrast, endergonic reactions absorb energy from their environment, just like inhalations draw in air. This absorbed energy is stored within the products of the reaction.
Spontaneity: The Path of Least Resistance
Imagine reactions as people walking along a path. Exergonic reactions flow down a spontaneous path, releasing energy and decreasing their free energy. They occur naturally without any external input, like a ball rolling downhill. Endergonic reactions, on the other hand, require energy input to proceed, making them non-spontaneous. They need an uphill push, just like hiking up a steep trail.
Entropy Change: The Dance of Chaos
Entropy, a measure of disorder, plays a significant role in spontaneity. Exergonic reactions typically increase entropy, creating more disordered products. Think of it as a messy room that becomes even more chaotic after a house party. Conversely, endergonic reactions often decrease entropy, resulting in more ordered products. It’s like cleaning up the messy room, bringing order to the chaos.
Gibbs Free Energy Change: Predicting Spontaneity
Gibbs free energy change (ΔG) is like a compass that helps us predict spontaneity. Negative ΔG values indicate that a reaction is spontaneous, while positive ΔG values suggest non-spontaneity. Exergonic reactions have negative ΔG values, while endergonic reactions have positive ΔG values. It’s a simple rule that provides valuable insights into reaction behavior.
Summary: A Comprehensive Overview
To summarize, exergonic reactions release energy, are spontaneous, increase entropy, and have negative ΔG values. Endergonic reactions, on the other hand, absorb energy, are non-spontaneous, decrease entropy, and have positive ΔG values. Understanding these differences is essential for predicting the spontaneity and directionality of chemical reactions, a cornerstone of chemistry and biology.