Eutectic Point: Understanding The Key To Material Properties And Applications
The eutectic point is a crucial concept in materials science, signifying the lowest temperature at which a liquid and two solid phases coexist. When a eutectic system solidifies, it undergoes a eutectic reaction, simultaneously solidifying both solid phases to form a characteristic eutectic mixture and structure. The eutectic point influences material properties like melting and solidification temperatures, strength, and corrosion resistance. Understanding the eutectic point is critical in designing and optimizing materials for diverse applications, including metallurgy, composites, and electronic devices.
Introduction to the Eutectic Point:
- Definition: Understanding the significance of the eutectic point in materials science.
The Eutectic Point: The Cornerstone of Materials Science
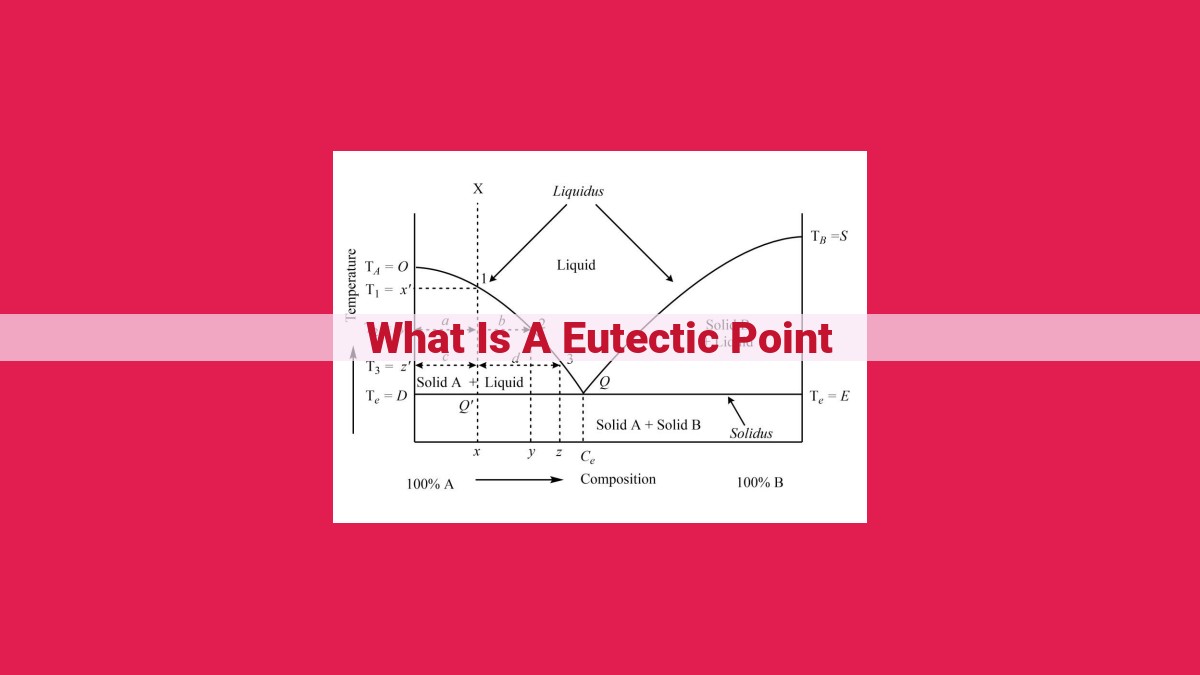
In the realm of materials science, there exists a fundamental concept that holds immense significance for understanding the behavior and properties of materials: the eutectic point. Just as the melting point defines the temperature at which a solid material transforms into a liquid, the eutectic point marks the lowest temperature at which a certain mixture of two or more components can coexist in a liquid-solid state.
Understanding the Eutectic Point
Imagine a binary system, a mixture of two elements or compounds. As you cool this mixture from a completely liquid state, each component tends to solidify at its own characteristic temperature, much like individual crystals forming in a solution. However, at a specific temperature, a unique phenomenon occurs: the liquid mixture suddenly transforms into a _eutectic mixture, a combination of solid phases that coexist seamlessly. This temperature is the system’s *eutectic point*.
Related Concepts
The eutectic system is the specific composition of two or more components that exhibit a eutectic point. This system typically forms when the components have limited solubility in each other in the solid state. The simultaneous solidification of the two solid phases in a eutectic mixture is known as the eutectic reaction. The resulting microstructure, consisting of fine, alternating layers of the two solid phases, is called the eutectic structure.
Understanding the Eutectic Point: The Lowest Temperature for Liquid-Solid Coexistence
In materials science, understanding the eutectic point is critical. It’s the lowest temperature at which a liquid and a solid can coexist in equilibrium.
Diving Deeper into the Eutectic Point
Consider a binary alloy system – two different metals mixed together. As you heat the mixture, it will eventually reach a point where it completely melts. However, if the mixture’s composition is just right, a curious phenomenon occurs: at a specific temperature, the liquid begins to freeze into two solid phases simultaneously. This eutectic point is like hitting the sweet spot, where the liquid and solid can happily coexist in perfect harmony.
Key Components of the Eutectic System
The eutectic system consists of:
- Eutectic Composition: The exact ratio of the two metals in the alloy at which the eutectic point occurs.
- Eutectic Temperature: The specific temperature at which the liquid and solid phases coexist.
- Eutectic Phase Diagram: A graphical representation showing the eutectic composition and temperature as a point on a phase diagram.
Significance of the Eutectic Point
The eutectic point significantly influences material properties. It affects melting and solidification temperatures, mechanical strength, and even corrosion resistance. Engineers and researchers harness this knowledge to design new materials with tailored properties for specific applications.
Applications of the Eutectic Point
Understanding the eutectic point is crucial in:
- Soldering and Welding: Manipulating the eutectic point allows for the creation of strong and reliable solder and weld joints.
- Phase Transformations: Controlling the eutectic point influences the timing and progression of solid-state phase transformations, enhancing material properties.
- Additives and Impurities: Understanding the effects of impurities on the eutectic point aids in optimizing alloy composition for improved performance.
The Eutectic Point: A Key Concept in Materials Science
Introduction:
The eutectic point, a fundamental concept in materials science, refers to the lowest temperature at which a liquid can coexist with two solid phases. Understanding this concept is crucial for materials scientists and engineers who develop and optimize materials for various applications.
Understanding the Eutectic Point:
The eutectic point represents a unique point on a phase diagram where the liquidus and solidus lines intersect. At this point, the liquid and solid phases can coexist in equilibrium, meaning they can exist together without any net change. The eutectic composition, which corresponds to the eutectic point, is a specific proportion of the components in the mixture.
Related Concepts:
Eutectic System: A eutectic system consists of two or more components that form a eutectic mixture. The components are usually metals or alloys that exhibit a eutectic behavior when combined.
Eutectic Mixture: When a eutectic system is cooled and solidifies, the liquid phase transforms simultaneously into two distinct solid phases at the eutectic temperature. This simultaneous solidification results in a fine-grained, interpenetrating microstructure, known as the eutectic mixture.
Eutectic Reaction: The phase transformation that occurs at the eutectic point is known as the eutectic reaction. This reaction represents the simultaneous formation of two solid phases from the liquid phase.
Eutectic Structure: The eutectic structure refers to the characteristic microstructure that forms in the eutectic mixture. It consists of a fine-grained, interpenetrating arrangement of the two solid phases, resulting from the simultaneous solidification during the eutectic reaction.
The Eutectic Point: A Critical Factor in Material Design and Performance
Understanding the eutectic point is crucial in materials science, as it holds significant influence on a material’s properties and applications. A eutectic point marks the lowest temperature at which a liquid and solid can coexist in equilibrium.
Influence on Material Properties
The eutectic point plays a pivotal role in determining a material’s melting and solidification characteristics. At the eutectic point, a material melts and solidifies simultaneously, leading to a sharp melting point and solidification temperature. This precise behavior allows for controlled and consistent processing, making materials with specific melting and solidification requirements possible.
Furthermore, the eutectic point affects a material’s mechanical properties. The formation of a eutectic mixture, composed of two solid phases, results in a unique microstructure that influences strength, hardness, toughness, and fracture behavior. By tailoring the eutectic composition, materials with optimized mechanical properties can be engineered.
Applications
The understanding of the eutectic point is essential in the design and optimization of materials for various applications. In metallurgy, eutectic alloys are widely used for soldering, brazing, and casting. The precise melting characteristics of eutectic alloys facilitate reliable joining of components and the production of castings with fine microstructures.
In the electronics industry, eutectic compositions are employed as solder for connecting electrical components. The well-defined melting point ensures proper bonding and prevents overheating, which is crucial for delicate electronic devices.
Furthermore, eutectic mixtures are utilized in thermal storage systems. The ability of a eutectic mixture to release or absorb a large amount of latent heat at a specific temperature makes it suitable for applications in heating and cooling systems, such as solar energy and building insulation.