Essential Conversion: Understanding And Converting Between Atm And Mm Hg
1 atm, the standard atmospheric pressure, equals 760 mm Hg, a pressure unit based on the height of a mercury column. Understanding the conversion factor and how to convert between these units is crucial in various fields, including meteorology, medicine, and engineering. Accurate pressure measurements contribute to scientific research and industrial processes, making the conversion between atm and mm Hg an essential skill.
Understanding Atmospheric Pressure and Standard Atmosphere (atm)
The Invisible Force that Shapes Our World
Imagine yourself floating in a vast ocean of air. Unseen, yet very real, this sea of gases exerts a constant force upon us at all times. This force, known as atmospheric pressure, is essential for life on Earth.
In the field of atmospheric sciences, atmospheric pressure measures the weight of the air column above a given point. The standard unit of atmospheric pressure is the standard atmosphere (atm), defined as the average sea-level pressure at a temperature of 15°C (59°F). This standardized value serves as a reference point for comparing and analyzing atmospheric conditions worldwide.
Why is Standard Atmosphere Important?
Understanding standard atmosphere is crucial for weather forecasting, aviation, and a wide range of scientific disciplines. Accurate measurements of atmospheric pressure help meteorologists predict weather patterns and storm intensity. Altitude, which is linked to atmospheric pressure, plays a critical role in determining aircraft performance and safe flight operations. In fact, various atmospheric gauges, like barometers, are essential tools used to monitor atmospheric pressure and make these vital predictions and calculations.
Millimeters of Mercury (mm Hg): A Pressure Measurement Unit
- Define mm Hg as a unit of pressure based on the height of a mercury column.
- Discuss its historical and current applications, such as in barometers and blood pressure measurement.
Millimeters of Mercury (mm Hg): The Age-Old Measure of Pressure
In the realm of pressure measurement, the unit of millimeters of mercury (mm Hg) holds a storied history and continues to find widespread application today. Its roots can be traced back centuries to the invention of the barometer, a device that measures atmospheric pressure. The barometer works on the principle that the height of a column of mercury is proportional to the surrounding pressure.
The mm Hg unit is defined as the pressure exerted by a column of mercury 1 millimeter high, under standard conditions of temperature and gravity. Its historical significance lies in its use in barometers, which were instrumental in the development of meteorology. Barometers enabled scientists to measure and track atmospheric pressure, leading to a deeper understanding of weather patterns and forecasting.
Beyond meteorology, the mm Hg unit has found diverse applications in other fields, notably medicine. In the medical context, mm Hg is used to measure blood pressure, a crucial indicator of cardiovascular health. Blood pressure readings are typically expressed in millimeters of mercury, representing the pressure exerted by the blood against the walls of the arteries.
Today, the mm Hg unit remains a widely used standard in various industries, including engineering, aviation, and manufacturing. It is commonly employed in pressure gauges, altimeters, and other precision instruments. Its widespread adoption owes to its accuracy, reliability, and historical precedence. Despite the introduction of newer pressure units, the mm Hg unit persists as a valuable tool in the fields of atmospheric science, medicine, and beyond.
The Conversion Factor: 1 atm = 760 mm Hg
- Explain the derivation and purpose of this conversion factor.
- Emphasize its importance in converting pressure measurements between different units.
The Conversion Factor: 1 atm = 760 mm Hg
In the realm of atmospheric pressure, two units reign supreme: atmospheres (atm) and millimeters of mercury (mm Hg). To navigate between these units seamlessly, we rely on a fundamental conversion factor: 1 atm = 760 mm Hg. This seemingly simple equation holds profound significance in atmospheric sciences and various fields.
The derivation of this conversion factor lies in the history of pressure measurement. In the 17th century, Evangelista Torricelli ingeniously devised the barometer, a device that measures atmospheric pressure. He used a glass tube filled with mercury and inverted it in a dish of mercury. The height of the mercury column in the tube balanced the pressure exerted by the atmosphere. This height, measured in millimeters, became the basis for the mm Hg unit.
A standard atmosphere (atm) is defined as the average atmospheric pressure at sea level, which is approximately equal to the pressure exerted by a column of mercury 760 mm high. Thus, the conversion factor 1 atm = 760 mm Hg was established.
The importance of this conversion factor cannot be overstated. It allows us to convert pressure measurements between atm and mm Hg, facilitating communication and understanding across different fields. Meteorologists use atm to describe air pressure patterns, while doctors and engineers often express pressures in mm Hg for blood pressure and fluid dynamics, respectively.
Converting Between Atm and mm Hg: A Practical Guide
In the realm of atmospheric sciences, precise pressure measurements are paramount, and two commonly used units are atm (standard atmosphere) and mm Hg (millimeters of mercury). Understanding how to convert between these units is essential for accurate data interpretation and scientific advancements.
Converting Atm to mm Hg
Step 1: Multiply by 760
To convert an atm value to mm Hg, simply multiply the atm value by 760. This conversion factor arises from the fact that 1 atm is defined as the pressure exerted by a mercury column with a height of 760 mm.
Example: Convert 2 atm to mm Hg:
2 atm x 760 mm Hg / atm = 1520 mm Hg
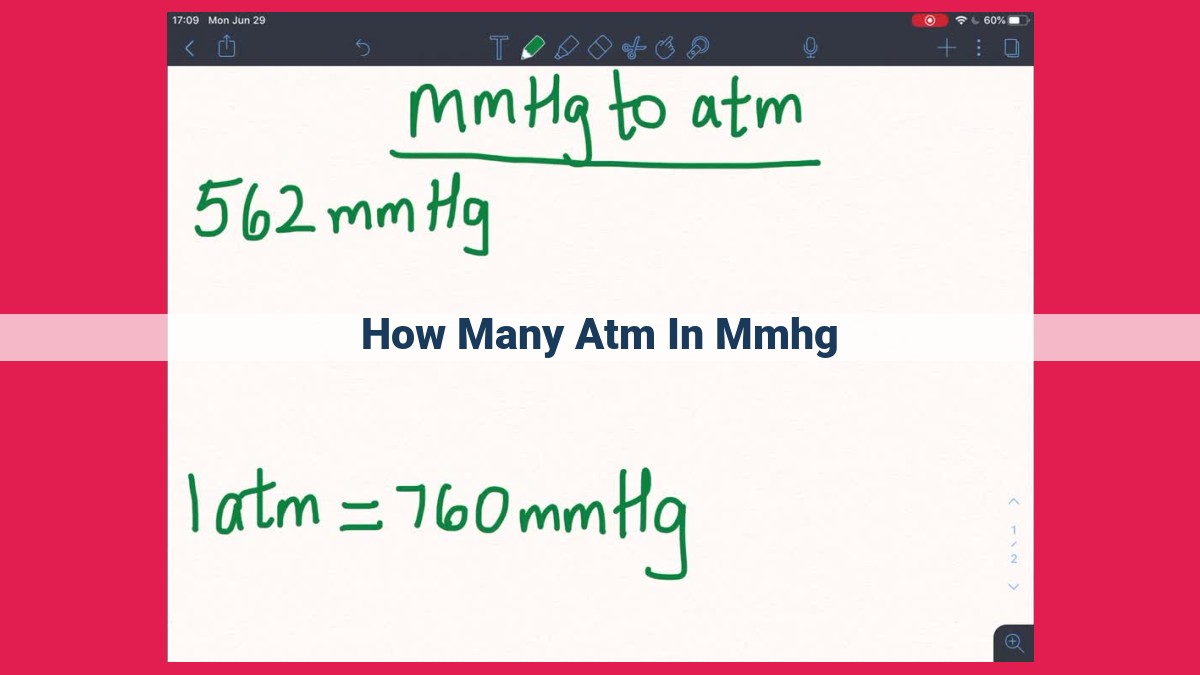
Converting mm Hg to Atm
Step 1: Divide by 760
To convert mm Hg to atm, divide the mm Hg value by 760. This follows from the inverse relationship between the two units.
Example: Convert 600 mm Hg to atm:
600 mm Hg ÷ 760 mm Hg / atm = 0.79 atm
Potential Pitfalls
- Unit Confusion: Always pay attention to the units of the values you are converting. Improper unit conversions can lead to erroneous results.
- Rounding Errors: When performing conversions, be cautious about rounding errors. It is generally advisable to use a calculator or software to ensure precision.
- Significant Figures: When reporting converted values, retain only the number of significant figures present in the original value to avoid misleading accuracy.
Applications of Pressure Units and Conversions
In the realm of science and industry, accurate pressure measurements are crucial for various applications. Understanding the conversion between atmospheres (atm) and millimeters of mercury (mm Hg) enables us to navigate these applications seamlessly.
Meteorology: Predicting the Weather’s Mood
Meteorologists rely on atmospheric pressure to forecast weather patterns. High pressure systems, characterized by elevated atm levels, often bring clear skies and stable conditions. Conversely, low atm readings indicate developing storm systems, prompting weather warnings. Converting pressure readings between atm and mm Hg helps meteorologists accurately predict weather events and keep communities informed.
Medicine: The Pulse of Healthcare
In the medical field, pressure measurements play a vital role. Blood pressure, measured in mm Hg, is a key indicator of cardiovascular health. Accurate mm Hg readings allow healthcare professionals to diagnose and monitor conditions such as hypertension and hypotension. Additionally, pressure units are used in anesthesia and respiratory therapy, ensuring patient safety and well-being.
Engineering: Shaping the World Around Us
Engineers harness pressure units to design and optimize systems in diverse industries. In aviation, atm measurements determine aircraft performance and flight safety. In construction, mm Hg gauges monitor water pressure, ensuring structural integrity and preventing leaks. Pressure conversions enable engineers to tailor their designs to specific applications, advancing technological progress.
Scientific Research: Uncovering Nature’s Secrets
Scientists rely on precise pressure measurements to study various phenomena. In oceanography, atm readings help determine the depth of bodies of water. In environmental science, mm Hg gauges measure the partial pressure of gases, such as carbon dioxide, in the atmosphere. These measurements contribute to our understanding of climate change, pollution, and other environmental concerns.
Industrial Processes: Precision in Manufacturing
Accurate pressure monitoring is essential in industrial settings. In manufacturing, atm gauges ensure optimal pressure for machinery operation and product quality. In the food industry, mm Hg measurements control the packaging and storage of perishable goods, preserving their freshness and extending their shelf life. Precise pressure conversions enable efficient and reliable industrial processes.
In conclusion, the conversion between atm and mm Hg serves a wide range of practical applications across diverse fields. By understanding this conversion, we unlock valuable insights into weather patterns, medical conditions, engineering innovations, scientific discoveries, and industrial efficiency. Accurate pressure measurements contribute to our understanding of the world and empower us to make informed decisions in various aspects of life.