Equilibrium Constant: Quantifying Reaction Behavior For Optimization
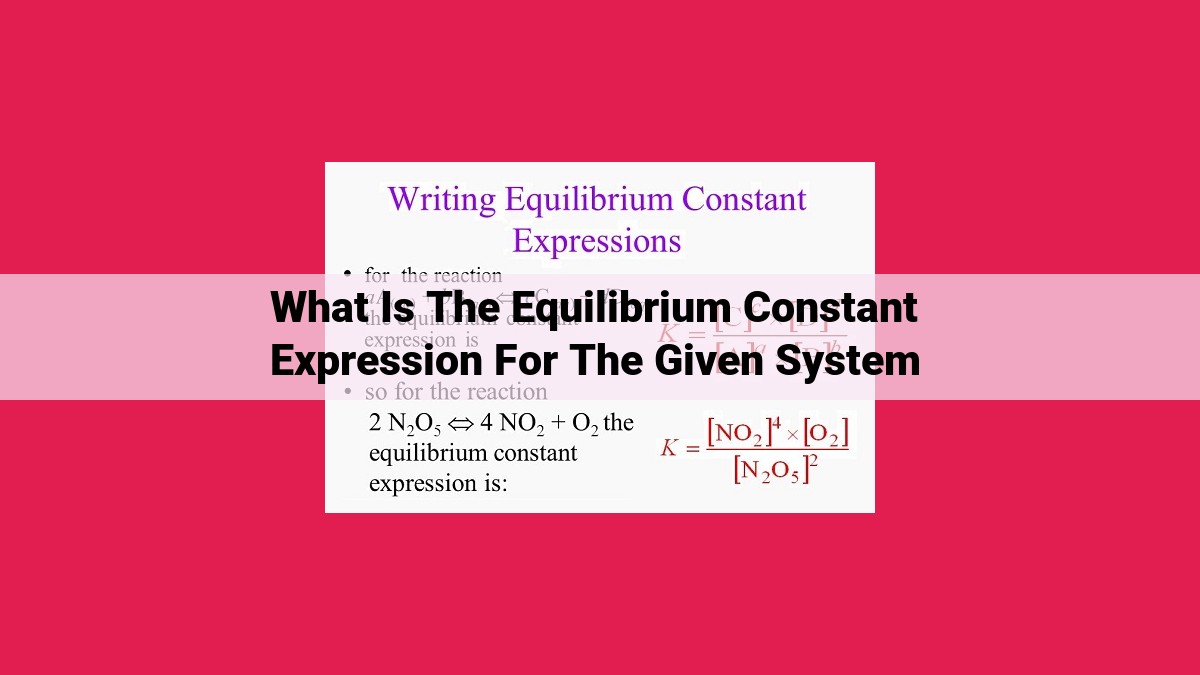
The equilibrium constant expression (K) quantifies the relationship between reactant and product concentrations at equilibrium, represented by [Reactants]^n/[Products]^m, where [ ] denotes concentration and n and m are stoichiometric coefficients. K determines the reaction direction and extent: K > 1 favors product formation, K < 1 favors reactant dominance. It is influenced by the balanced equation, reactant/product concentrations, and temperature. Standard states and activity coefficients are used to adjust K values in non-ideal solutions. K aids in predicting reaction behavior, assessing feasibility, and optimizing reaction conditions.
Understanding the Equilibrium Constant: A Foundation for Predicting Chemical Reactions
In the realm of chemistry, understanding the equilibrium constant is paramount. Equilibrium is a dynamic state where the forward and reverse reactions of a chemical process occur at equal rates, with no net change in the concentrations of reactants and products. The equilibrium constant, denoted as K, provides a crucial insight into the behavior of these reactions.
Defining the Equilibrium Constant (K)
The equilibrium constant is a numerical value derived from the equilibrium constant expression, which reflects the ratio of the concentrations of products to the concentrations of reactants at equilibrium. Each concentration is raised to a power equal to its stoichiometric coefficient balanced in the chemical equation.
Importance of K in Predicting Reaction Behavior
The equilibrium constant plays a vital role in predicting the direction and extent of reactions. If K is greater than 1, the reaction proceeds rightward, favoring the formation of products. Conversely, if K is less than 1, the reaction proceeds leftward, favoring the formation of reactants. Furthermore, the magnitude of K (either large or small) indicates the extent to which the reaction proceeds towards completion.
Factors Influencing the Equilibrium Constant
Understanding the equilibrium constant (K) is crucial for predicting the direction and extent of chemical reactions. Several factors influence its value, shedding light on reaction behavior.
Reaction Quotient (Q)
The reaction quotient (Q) is a dimensionless quantity calculated using the concentrations of reactants and products at a specific moment in a reaction. It measures the relative amounts of reactants and products present and helps determine the direction in which the reaction will proceed.
Balanced Chemical Equation
The balanced chemical equation provides the stoichiometric information necessary to calculate K. The coefficients in the equation represent the number of moles of each reactant and product involved in the reaction. These coefficients determine the exponents used in the equilibrium constant expression.
Reactant and Product Concentrations
The concentrations of reactants and products directly affect the numerical value of K. According to the law of mass action, the equilibrium constant is proportional to the product of the product concentrations divided by the product of the reactant concentrations, raised to their respective stoichiometric coefficients.
Temperature
Temperature strongly influences the equilibrium constant. Generally, increasing temperature shifts the equilibrium towards products for exothermic reactions (heat released) and towards reactants for endothermic reactions (heat absorbed). This effect is described by the van’t Hoff equation.
These factors, when considered together, paint a comprehensive picture of the equilibrium position and reaction behavior. By examining the reaction quotient, balanced chemical equation, and temperature dependence, chemists can make informed predictions about the feasibility and direction of chemical reactions.
Standard State and Activity Coefficients: Unraveling the Complexity
The Standard State: A Reference Point in Chemical Equilibrium
When chemists analyze equilibrium reactions, they often refer to the concept of a standard state. This imaginary state serves as a crucial benchmark to which all other states are compared. By convention, the standard state for a substance is defined as its pure form at a temperature of 298 K (25 °C) and a pressure of 1 bar. In this standard state, the activity of a substance is considered to be unity (1), providing a stable reference point for understanding its behavior in other states.
Activity Coefficients: Adjusting for Non-Ideal Solutions
In real-world scenarios, solutions are often not ideal. This means that the behavior of a substance in a solution can deviate from its behavior in an ideal state. To account for these deviations, chemists introduce the concept of activity coefficients (γ). These coefficients represent the ratio of the actual activity of a substance to its activity in the standard state.
Activity coefficients are particularly important when dealing with non-ideal solutions, where interactions between solute particles can significantly affect their behavior. These interactions can either enhance or diminish the activity of a substance compared to its standard state. By incorporating activity coefficients into equilibrium calculations, chemists can accurately predict the equilibrium constant (K) and the extent of reactions in non-ideal solutions.
Determining the Equilibrium Constant: Experimental and Theoretical Approaches
Comprehending the equilibrium constant (K) is essential for predicting chemical reaction outcomes. Determining K involves two primary methods: experimental measurements and theoretical calculations.
Experimental Determination of K
To measure K experimentally, chemists analyze reaction data. They monitor the concentrations of reactants and products over time until the system reaches equilibrium. By substituting these values into the equilibrium constant expression, they can directly determine K. This method is particularly valuable for simple reactions with well-behaved systems.
Theoretical Calculations of K
Alternatively, K can be calculated theoretically using thermodynamic principles. The Gibbs free energy change (ΔG) for a reaction is related to K through the equation ΔG = -RTlnK, where R is the gas constant and T is the temperature. By determining ΔG from thermochemical data or spectroscopic measurements, chemists can indirectly calculate K.
This approach offers several advantages. It allows for K determination under various conditions, including high temperatures or pressures, where direct measurements may be impractical. Theoretical calculations can also provide insights into the energetic factors driving the equilibrium position.
Combining Methods for a Comprehensive Understanding
While experimental measurements provide direct confirmation of K, theoretical calculations offer additional insights and broaden the range of applicable conditions. By combining both approaches, chemists gain a comprehensive understanding of equilibrium constants and their implications for chemical reactions.
The Power of the Equilibrium Constant: Predicting Reaction Behavior
The equilibrium constant (K) is a crucial tool in chemistry, playing a pivotal role in understanding and predicting the behavior of chemical reactions. It holds the key to unlocking the secrets of chemical transformations.
K and the Direction of Reactions
The value of K provides invaluable insights into the direction a reaction will take. A K greater than 1 indicates that the reaction favors the formation of products. Conversely, a K less than 1 signifies a preference for reactants. This knowledge enables chemists to anticipate the outcome of a reaction before it even occurs.
K as an Indicator of Completeness and Reversibility
K also sheds light on the completeness and reversibility of reactions. A large K suggests that the reaction proceeds nearly to completion, with minimal reactants remaining. On the other hand, a small K indicates a reversible reaction, where reactants and products continuously interconvert.
By understanding the concept of the equilibrium constant, we gain the ability to foresee the fate of chemical reactions. This knowledge empowers chemists to design and control chemical processes, optimizing their efficiency and targeting desired outcomes.
Understanding the Equilibrium Constant: A Guide to Predicting Reaction Behavior
Applications of the Equilibrium Constant
The equilibrium constant (K) is a powerful tool that not only helps us understand the direction and extent of reactions but also has wide-ranging applications in chemistry and beyond.
Determining the Feasibility of Chemical Processes:
K plays a crucial role in assessing the feasibility of chemical processes. By examining the value of K, chemical engineers and industrial chemists can determine whether a particular reaction is thermodynamically favorable or not. Reactions with a large equilibrium constant (K >> 1) typically proceed to completion, making them ideal candidates for industrial-scale production.
Optimizing Reaction Conditions:
Understanding K allows chemists to optimize reaction conditions, such as temperature, pressure, and concentration, to maximize the yield of desired products. By manipulating these factors, research chemists can fine-tune reactions and increase production efficiency.
Understanding Complex Systems:
The equilibrium constant is essential in studying complex systems, such as biological processes and environmental chemistry. By understanding the equilibrium constants governing these systems, scientists can develop models to predict their behavior and identify potential interventions or environmental impacts.
In summary, the equilibrium constant is a versatile tool that provides valuable insights into reaction behavior and has numerous applications across various fields. It empowers chemists, engineers, and scientists to harness the power of thermodynamics to optimize processes, understand complex systems, and drive innovation in diverse domains.