Enzymes: Unlocking The Power Of Biochemical Reactions
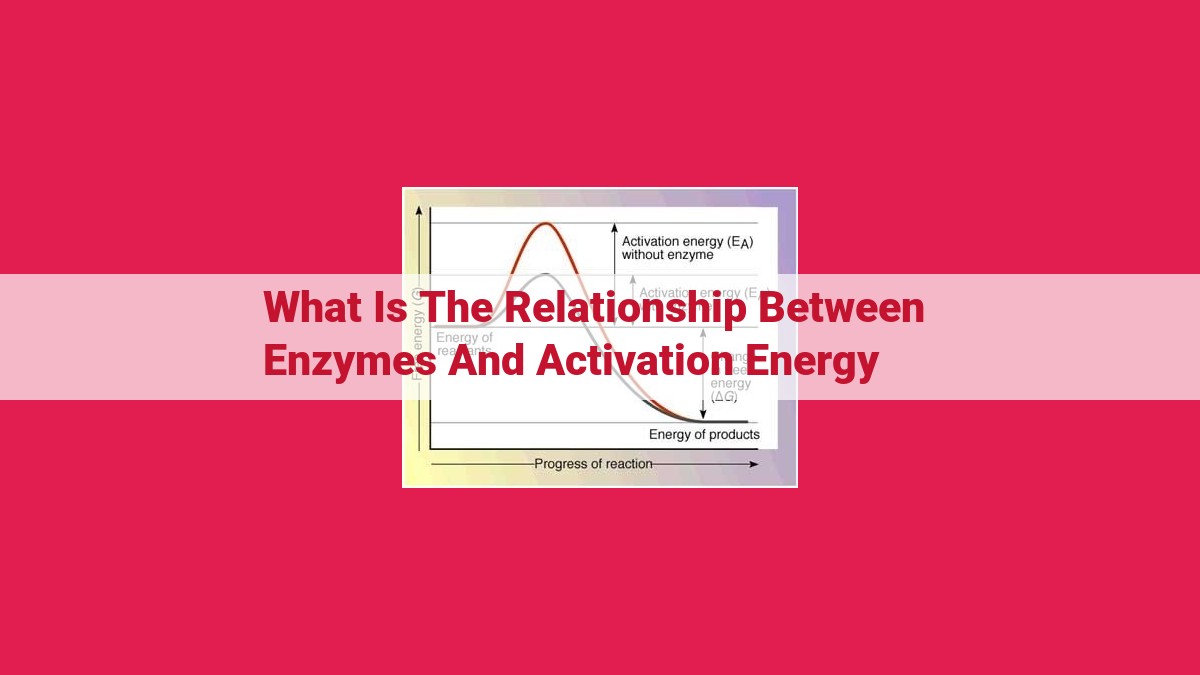
Enzymes are biological catalysts that reduce the activation energy, the barrier hindering chemical reactions. They achieve this by providing an alternative reaction pathway with a lower activation energy, facilitated by the enzyme’s active site and cofactors. The enzyme-substrate complex formation stabilizes the transition state, further lowering activation energy and accelerating the transformation of reactants to products. This enzyme-mediated reduction in activation energy is crucial for efficient cellular processes and maintaining life, as it enables reactions to occur at physiological temperatures and speeds.
Enzymes: The Unsung Heroes of Life
In the intricate tapestry of life, enzymes play a pivotal role, orchestrating the countless chemical reactions that sustain our very existence. These biological catalysts tirelessly reduce activation energy, the barrier that hinders chemical reactions, enabling them to proceed at remarkable speeds. Without these molecular maestros, life as we know it would cease to exist.
Enzymes are highly specialized proteins, each with a unique active site that accommodates a specific substrate molecule. This interaction triggers a cascade of events that ultimately converts the substrate into a product. This seemingly effortless process is a testament to the enzyme’s ability to bypass the innate resistance of molecules to change, allowing reactions to occur at rates that would otherwise be impossible.
Delving into the Concept of Activation Energy
Imagine a chemical reaction as a mountain that needs to be climbed. The height of the mountain represents the activation energy, the energy required to initiate the reaction. Without this initial push, the reaction remains stagnant at the base of the peak. This is where enzymes step in, providing an alternative path, like a winding mountain trail, that significantly lowers the activation energy required to reach the summit.
Understanding Activation Energy: The Barrier to Chemical Reactions
In the heart of chemical reactions lies a hidden hurdle known as activation energy, the energy that must be overcome for a reaction to proceed. Imagine it as a mountain you must climb before reaching the summit of a successful reaction.
The climb to the summit represents the process of transforming reactants into products. However, this journey is not always smooth. The peak of the mountain, known as the transition state, requires a significant energy input to overcome, like pushing a boulder uphill.
To ease this arduous climb, nature has crafted a secret ally: enzymes. These molecular helpers provide an alternative pathway, a secret tunnel that leads to the summit without the need for excessive energy. Enzymes do this by forming a cozy union with the reactants, creating an enzyme-substrate complex. This snug embrace stabilizes the transition state, making it easier for the reactants to transform into products.
Without enzymes, many biological reactions would be as arduous as hiking Mount Everest without a sherpa. Enzymes lower the activation energy, providing a smoother path for countless chemical reactions essential to life. From digestion to metabolism, enzymes are the unsung heroes that make our bodies tick.
Enzymes and Activation Energy: Unlocking the Secrets of Chemical Reactions
In the intricate dance of life, chemical reactions play a pivotal role. However, nature faces a hurdle: the activation energy barrier—the energy required to kick-start a reaction. Enter enzymes, the master catalysts of life, who possess the secret of bypassing this energy roadblock.
Enzymes, akin to molecular magicians, offer an alternative reaction pathway—a shortcut that lowers the activation energy needed. They create a more favorable path for reactants to transform into products, like guiding lost travelers through a secret tunnel.
At the heart of enzyme activity lies the active site, a tailored cavity where the substrate (the reactant molecule) finds its perfect fit. Like a lock and key, the active site is designed to accommodate specific substrates, ensuring precision and efficiency.
Cofactors, the enzyme’s trusty assistants, come in various forms, from metal ions to vitamins. They lend their support to the active site, helping to stabilize the substrate and further reduce activation energy. Imagine a mechanic using a specialized tool to unlock a stubborn nut—cofactors are just that vital.
Enzyme activity is a delicate balance, influenced by factors like temperature and pH. Alterations in these factors can disrupt enzyme activity, leading to cellular dysfunction and even disease. Understanding enzyme activity is thus crucial for maintaining health and unraveling the mysteries of life’s chemical tapestry.
The Enzyme-Substrate Complex
- Explain the formation and importance of the enzyme-substrate complex.
- Discuss the concept of induced fit and how it enhances enzyme specificity.
The Enzyme-Substrate Complex: A Molecular Dance
In the intricate ballet of biochemical reactions, enzymes take center stage as they orchestrate the transformation of molecules. One crucial step in this dance is the formation of the enzyme-substrate complex – a temporary embrace that sets the stage for catalysis.
Birth of the Complex
As an enzyme encounters its substrate, the molecule it’s destined to transform, a delicate interaction ensues. The enzyme’s active site, a precisely sculpted cavity, complements the substrate’s shape and chemical properties. Like a lock and key, they fit together with exquisite specificity, forming the enzyme-substrate complex.
Induced Fit: A Tailored Embrace
Remarkably, the enzyme-substrate complex is not static. The enzyme’s active site undergoes subtle changes to accommodate the substrate, ensuring optimal contact and reactivity. This phenomenon, known as induced fit, further enhances the enzyme’s specificity, preventing other molecules from hijacking the catalytic process.
Enhancing Specificity
The enzyme-substrate complex is the foundation for the enzyme’s catalytic prowess. The precise fit between enzyme and substrate allows the enzyme to orient the substrate in the most favorable position for the reaction. It also prevents unwanted side reactions, ensuring that only the desired product is formed.
The enzyme-substrate complex is a pivotal step in the enzymatic dance. Through its formation and tailored fit, enzymes exhibit remarkable specificity, transforming substrates into products with unmatched precision. This molecular ballet underpins the myriad of chemical reactions that sustain life, making enzymes indispensable orchestrators in the symphony of biological systems.
Transition State and Enzyme Stabilization
Catalysts play a pivotal role in chemical reactions, enabling them to proceed at a much faster rate. Enzymes, nature’s catalysts, excel in this regard, drastically lowering the activation energy required for reactions to occur.
Enzymes achieve this by providing an alternative pathway for reactions. This pathway features a transition state, an unstable, high-energy intermediate that forms during the transformation of reactants to products.
Stabilization by Enzymes
Enzymes stabilize this transition state, effectively lowering its energy level. This makes the transition state more accessible, reducing the overall activation energy required for the reaction. Enzymes achieve this stabilization through various mechanisms.
One mechanism involves the active site, a specific region of the enzyme that binds to and interacts with the substrate, the molecule undergoing the reaction. The active site’s structure is perfectly tailored to the shape and charge of the transition state, creating an ideal environment for its stabilization.
Facilitating Transformation
Additionally, enzymes may employ cofactors, non-protein molecules that assist in the catalytic process. Cofactors can stabilize the transition state through ionic or hydrogen bonding interactions, further reducing activation energy.
By creating a favorable environment for the transition state, enzymes facilitate the transformation of reactants to products. This catalytic power is essential for life, as it enables the complex chemical reactions necessary for cellular function and metabolism.
Mechanisms of Activation Energy Reduction: Enzymes Unlocking Chemical Reactions
In the realm of biology, enzymes reign supreme as the master catalysts, orchestrating the intricate dance of chemical reactions that define life. Their unmatched ability to accelerate reaction rates lies in their enchanting power to lower activation energy, the formidable barrier that impedes reactions from taking place.
How Enzymes Overcome the Activation Energy Barrier
Enzymes achieve this remarkable feat through an arsenal of ingenious mechanisms, each a testament to nature’s exquisite design:
Providing an Alternative Pathway
Enzymes offer a shortcut to chemical reactions, providing an alternative pathway that bypasses the treacherous energy hurdle. By creating a new, less arduous route, enzymes dramatically reduce the amount of energy required for reactions to proceed.
Stabilizing the Transition State
Reactions often encounter a precarious state known as the transition state, a fleeting moment when the reactants are poised to transform into products. Enzymes step in as stabilizing forces, cradling the transition state and lowering its energy barrier. This gentle embrace makes reactions far more likely to occur and complete.
Orienting Substrates
Enzymes are meticulous matchmakers, bringing substrates—the reactants in a reaction—together in an optimal orientation. This precise alignment ensures that the substrates perfectly interact, maximizing the chances of a successful reaction.
Implications of Reduced Activation Energy
The impact of reduced activation energy is profound. By lowering this barrier, enzymes dramatically accelerate reactions, enabling vital biological and chemical processes to take place at a pace that sustains life. These reactions govern everything from nutrient digestion to immune responses, underpinning our very existence. Conversely, alterations in enzyme activity can have far-reaching consequences, potentially leading to cellular dysfunction or even disease.
Enzymes stand as indispensable partners in the symphony of life. Their ability to lower activation energy by providing alternative pathways, stabilizing transition states, and orienting substrates underscores their crucial role in catalysing reactions that drive cellular processes. Without the magic of enzymes, life as we know it would simply not exist.